Deranged Calciotropic Hormonal Activity
Parathyroid Hormone Excess or Deficiency
Primary hyperparathyroidism is due to increased intrinsic activity of the parathyroid gland, altering the secretion of parathyroid hormone (PTH), in the absence of a known or recognized stimulus affecting calcium homeostasis.
Primary hyperparathyroidism is due to increased intrinsic activity of the parathyroid gland, altering the secretion of parathyroid hormone (PTH), in the absence of a known or recognized stimulus affecting calcium homeostasis. Primary hyperparathyroidism is a common endocrine disorder, but there are some rare forms of this disease such as: family isolated hyperparathyroidism, hyperparathyroidism familial not isolated, parathyroid cancer, hyperparathyroidism-jaw tumor syndrome, and ectopic hyperparathyroidism associated to carcinomas.
Hyperparathyroidism familial not isolated is associated to multiple endocrine neoplasias, such as multiple endocrine neoplasia (MEN) type 1, 2A and 4.
Primary hyperparathyroidism is the most common feature of MEN1. Patients may have asymptomatic hypercalcemia, nephrolithiasis, osteitis fibrosa cystica, vague symptoms associated with hypercalcemia, or occasionally peptic ulcers. The hypercalcemia is usually mild, and severe hypercalcemia resulting in crisis or parathyroid cancers is rare. Primary hyperparathyroidism associated with MEN1 is characterized by: early age at onset, greater reduction in bone mineral density than primary hyperparathyroidism not associated with MEN1, and an equal male/female ratio (1:1 vs. 1:3). All parathyroid glands may be affected. Subtotal or total parathyroidectomy with heterotopic autotransplantation in patients with MEN1 is the definitive treatment.
Primary hyperparathyroidism develops in 20% to 30% of patients with MEN2A. The mean age of onset is 36 years. It is the first manifestation of the syndrome in less than 5% of cases. The hypercalcemia is usually mild, and most of patients are asymptomatic. The size of the parathyroid glands may vary greatly.
Subtotal parathyroidectomy with preservation of a small remnant of one gland or total parathyroidectomy with heterotopic autotransplantation are the treatment options.
MEN4 is characterized by the occurrence of parathyroid and anterior pituitary tumors in possible association with tumors of the adrenals, kidneys, and reproductive organs.
MEN type 1
(OMIM phenotype number #131100)
Gene: MEN1 gene, 11q13.1 (OMIM gene/locus number *613733).
MEN type 2A
(OMIM phenotype number #171400)
Gene: RET gene, 10q11.21 (OMIM gene/locus number +164761).
MEN type 4
(OMIM phenotype number #610755 OMIM phenotype number #610755)
Gene: CDKN1B gene, 12p13.1 (OMIM gene/locus number *600778).
Phenotype
Primary hyperparathyroidism: Endocrine disorder resulting from a persistent hypercalcemia supported by an inadequate secretion of PTH; rarely it occurs in familial syndromes. Systemic signs: renal (polyuria, hypercalciuria, nephrolithiasis) skeletal, neuromuscular (myopathy, chondrocalcinosis, arthritis), central nervous system (fatigue, cognitive changes), gastrointestinal (peptic ulcer, pancreatitis), cardiovascular (hypertension, reduction QT interval), bone-specific signs: osteoporosis with a reduction of bone mineral density mainly of the cortical bone, osteitis fibrosa cystica in severe cases (subperiostal resorption of the phalange, salt and pepper appearance of the skull, bone cysts, brown tumors of the long bones).
MEN 1: Parathyroid adenoma (90%) + Enteropancreatic tumor (30–70%): gastrinoma (40%), insulinoma (10%), nonfunctioning and PPoma (20–55%), glucagonoma (1%), VIPoma (1%); pituitary adenoma (30–40%): prolactinoma (20%), somatotropinoma (10%), corticotropinoma (5%), nonfunctioning (5%); associated tumors: adrenal cortical tumor (40%), pheochromocytoma (1%), bronchopulmonary NET (neuroendocrine tumor) (2%), thymic NET (2%), gastric NET (10%), lipomas (30%), angiofibromas (85%), collagenomas (70%), meningiomas (8%).
MEN 2A: Parathyroid adenoma (20–30%) + medullary thyroid carcinoma (MTC) (90%), pheochromocytoma (50%).
MEN 4: Parathyroid adenoma + Enteropancreatic endocrine tumors, anterior pituitary tumors, in possible association with tumors of the adrenals, kidneys, and reproductive organs (reproduction organ tumors: e.g. testicular cancer, neuroendocrine cervical carcinoma).
Main biochemical alterations
MEN 1: High Ca, low Pi, high Ur Pi, high Ur Ca, high PTH + other pituitary and/or enteropancreatic hormone alterations.
MEN 2A: High Ca, low Pi, high Ur Pi, high Ur Ca, high PTH + high calcitonin (always), high catecholamine/catecholamine metabolites.
MEN 4: High Ca, low Pi, high Ur Pi, high Ur Ca, high PTH + other pituitary and/or enteropancreatic hormone alterations.
Images
Fig 1. Parathyroid imaging in MEN 1: ultrasonography (US) and computed tomography (CT). (a) Parathyroid adenoma. US image of the neck demonstrates the typical appearance of a parathyroid adenoma in MEN 1: a well-defined, oval hypoechoic mass posterior to the thyroid gland. (b) Parathyroid adenoma in a different patient. Contrast material–enhanced CT scan of the neck shows a right inferior parathyroid adenoma (arrow).
Fig 2. Parathyroid imaging in MEN: MR imaging. (a) Parathyroid adenoma. Coronal T2-weighted MR image shows a right parathyroid adenoma with homogeneous high signal intensity (arrow) in the neck. (b, c) Parathyroid adenoma in a patient who presented with persistent hypercalcemia. The patient had previously undergone subtotal parathyroidectomy. (b) Coronal T1-weighted MR image shows a bilobed, low-signal-intensity, left mediastinal parathyroid adenoma (arrow) adjacent to the aortic arch. (c) Corresponding technetium 99m (99mTc)–sestamibi (MIBI) scintigram shows increased radiotracer uptake within the adenoma.
Reproduced from Scarsbrook AF, Thakker RV, Wass JA, et al., Multiple endocrine neoplasia: spectrum of radiologic appearances and discussion of a multitechnique imaging approach, Radiographics 2006;26:433-51 with permission from The Radiographical Society of North America.
Other resources:
Hyperparathyroidism-jaw tumor syndrome (HPT-JT) is a rare, autosomal-dominant disease, secondary to germline-inactivating mutations of the tumor suppressor gene CDC73 (prevalence unknown).
(OMIM phenotype number #145001)
Hyperparathyroidism-jaw tumor syndrome (HPT-JT) is a rare, autosomal-dominant disease, secondary to germline-inactivating mutations of the tumor suppressor gene CDC73 (prevalence unknown). This diseases is characterized by benign and malignant parathyroid tumours, ossifying jaw tumours, various cystic and neoplastic renal abnormalities and benign and malignant uterine tumours. Primary hyperparathyroidism is the main finding of HPT-JT syndrome (up to 95% of individuals with HPT-JT). HPT-JT-associated primary hyperparathyroidism is often caused by a single benign parathyroid adenoma, and a second parathyroid tumor may occur after appearance of the first tumor. In 10%-15% of cases, primary hyperparathyroidism is caused by parathyroid carcinoma, and non-functioning parathyroid carcinomas have also been described. The onset of primary hyperparathyroidism in HPT-JT syndrome is in late adolescence or early adulthood. Individuals affected by primary hyperparathyroidism may be asymptomatic or present nephrolithiasis, reduced bone mass, fatigue, muscle weakness, bone or joint pain, and constipation.
The surgery is the typical approach to primary hyperparathyroidism in HPT-JT syndrome. In the case of a single parathyroid tumor, it has been suggested a minimally invasive surgical approach to remove the parathyroid tumor followed by close, monitoring for recurrent primary hyperparathyroidism.
Gene
HRPT2 (CDC73) gene, 1q31.2 (OMIM gene/locus number *607393).
Phenotype
Fibro-osseous tumors of the jaw, benign and/or malignant lesions over the course of the lifetime (most common: Wilms’ tumor, papillary renal carcinoma), polycystic kidney disease.
Main biochemical alterations
High Ca, low Pi, high Ur Pi, high Ur Ca, high PTH.
Images
Fig. Immunohistochemical expression. a; Weak parafibromin immunohistochemical cytoplasmic expression, with absence of nuclear positivity (40×); b surrounding control tissue showing normal expression of the protein.
Reproduced from Cell Oncol (Dordr), A novel CDC73 gene mutation in an Italian family with hyperparathyroidism-jaw tumour (HPT-JT) syndrome, 2014;37:281-8, Chiofalo MG, Sparaneo A, Chetta M, et al., with permission of Springer.
Familial isolated primary hyperparathyroidism (FIHP) is characterized by primary hyperparathyroidism not associated with other features. FIHP is essentially a diagnosis of exclusion.
(OMIM phenotype number #145000)
Familial isolated primary hyperparathyroidism (FIHP) is characterized by primary hyperparathyroidism not associated with other features. FIHP is essentially a diagnosis of exclusion. The clinical picture is characterized by familial primary hyperparathyroidism in the absence of clinical, radiological or biochemical features for diagnoses of MEN 1, MEN2A, HPT-JT, or FHH. The incidence is approximately 1% of all cases of primary hyperparathyroidism.
It has been described individuals affected by FIHP with a germline CDC73 pathogenic variant, presenting a more severe clinical presentation and younger age of onset than individuals with FIHP without CDC73 pathogenic variant. In most cases, these subjects with FIHP have at least one family member with a histopathologic diagnosis of parathyroid carcinoma and/or had a parathyroid adenoma with atypical or cystic features.
In approximately 20% of subjects with FIHP, MEN1 germline pathogenic variants have been described (see also MEN), and in 14%-18% of individuals with FIHP, heterozygous CASR pathogenic variants have also been reported.
Histologically, FIHP manifests itself as parathyroid chief cell hyperplasia, single and multiple gland adenoma as well as parathyroid carcinoma.
Genes
- MEN1 gene, 11q13.1 (OMIM gene/locus number #613733).
- CaSR gene, 3q21.1 (OMIM gene/locus number #601199).
- HRPT2 (CDC73) gene, 1q31.2 (OMIM gene/locus number #607393).
Phenotype
Endocrine disorder resulting from a persistent hypercalcemia supported by an inadequate secretion of PTH. Systemic signs: renal (polyuria, hypercalciuria, nephrolithiasis) skeletal, neuromuscular (myopathy, chondrocalcinosis, arthritis), central nervous system (fatigue, cognitive changes), gastrointestinal (peptic ulcer, pancreatitis), cardiovascular (hypertension, reduction QT interval), bone-specific signs: osteoporosis with a reduction of bone mineral density mainly of the cortical bone, osteitis fibrosa cystica in severe cases (subperiostal resorption of the phalange, salt and pepper appearance of the skull, bone cysts, brown tumors of the long bones).
Main biochemical alterations
High Ca, low Pi, high Ur Pi, high Ur Ca, high PTH.
Neonatal severe hypoparathyroidism is a rare autosomal recessive disorder of calcium homeostasis. NSHPT occurs in the first 6 months of life, but is often discovered in the first few weeks postnatally. Homozygous CASR germline pathogenic variants are classically associated with NSHPT.
(OMIM phenotype number #239200)
Neonatal severe hyperparathyroidism is a rare autosomal recessive disorder of calcium homeostasis. NSHPT occurs in the first 6 months of life, but is often discovered in the first few weeks postnatally. Homozygous CASR germline pathogenic variants are classically associated with NSHPT. The CaSR gene encodes the calcium-sensing receptor (CaSR), a G protein-coupled receptor that is highly expressed in the parathyroid and kidney. The inactivating mutations of CaSR gene reduce the sensitivity of the CaSR to extracellular calcium, with consequent increased parathyroid secretion of PTH and decreased renal excretion of calcium. NSHPT is characterized by parathyroid hyperplasia, marked and symptomatic PTH-dependent hypercalcemia, relative hypocalciuria, and bone fragility. Early radiologic features include: bony demineralization, pathologic fractures of long bones and ribs, subperiosteal resorption, rib fractures and rachitic changes. Infants with NSHPT often exhibit polyuria, dehydration and hypotonia associated with a history of failure to thrive, respiratory distress, irritability, lethargy, constipation, and delayed neuropsychological development. All these clinical manifestations are more evident if HPT is not promptly treated. NSHPT can be fatal if partial or total parathyroidectomy is not carried out within the first several weeks of life, with a good short-term prognosis after surgery and rapid involution of bony abnormalities.
Intravenous aminobisphosphonates are used in NSHPT to control severe hypercalcemia prior parathyroidectomy or as a rescue therapy to stabilise life-threatening demineralization. Recently, Gannon AW et al. have described the first use of cinacalcet as monotherapy for severe hypercalcemia in a newborn affected by NSHPT, showing a rapid and durable response to cinacalcet.
Gene
CaSR gene, 3q21.1 (OMIM gene/locus number +601199).
Phenotype
Life-threatening, severe osteoporosis.
Main biochemical alterations
Extremely high Ca, high Ur Ca, low Pi, high Ur Pi, high PTH.
Images
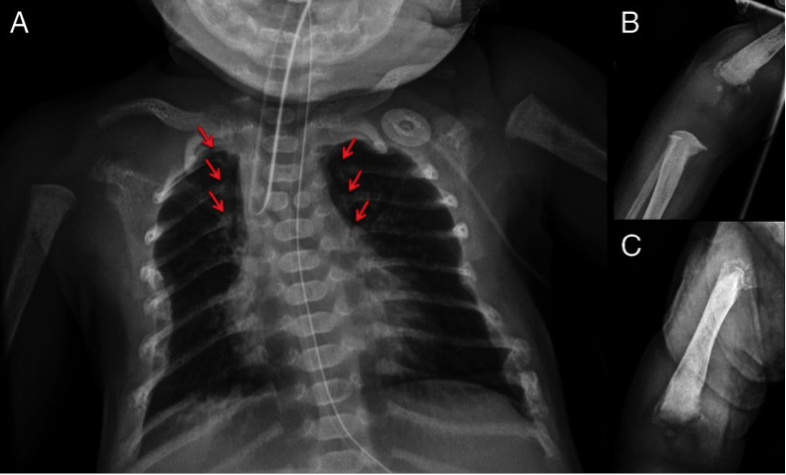
Fig. Radiographs of the patient affected by NSHPT demonstrating diffuse demineralization (A), multiple rib fractures (arrows), and chondrodystrophy of the distal humerus (B) and femur (C). A butterfly vertebrae was also noted on the chest radiograph.
Reproduced from J Clin Endocrinol Metab 99;1:7-11 under the terms of the Creative Commons Attribution License (CC BY).
Familial hypocalciuric hypercalcemia (HHC) is a benign condition associated with hypercalcemia, low urinary calcium excretion (assessed via a calcium/creatinine clearance ratio), normal to minimally elevated PTH levels, and hypermagnesemia.
Type I
Familial hypocalciuric hypercalcemia type I (HHC1) (OMIM phenotype number #145980)
Type II
Familial hypocalciuric hypercalcemia type II (HHC2) (OMIM phenotype number #145981)
Type III
Familial hypocalciuric hypercalcemia type III (HHC3) (OMIM phenotype number #600740)
Familial hypocalciuric hypercalcemia (HHC) is a benign condition associated with hypercalcemia, low urinary calcium excretion (assessed via a calcium/creatinine clearance ratio), normal to minimally elevated PTH levels, and hypermagnesemia.
HHC is usually asymptomatic but in some cases: fatigue, weakness, excessive thirst and concentration problems have been reported. Some adults present also pancreatitis, chondrocalcinosis and premature vascular calcification.
Usually, this condition does not require specific therapy, and responds poorly to parathyroidectomy.
HCC is a genetically heterogeneous disorder with three variants: types 1, 2, and 3.
In adult subjects, inactivating mutations of CaSR gene have been described in HHC type I (65% of cases), autosomal dominant disease, resulting in a reduction in the sensitivity of parathyroid and renal cells to calcium levels so hypercalcemia is perceived as normal.
HHC type II is caused by heterozygous mutation, with loss of function in the GNA11 gene, encoding G-protein subunit α11 (Gα11), involved in calcium-sensing receptor signaling.
HHC type III is caused by heterozygous mutation in the AP2S1 gene, which results in altered calcium-sensing receptor endocytosis.
Genes
- CaSR gene, 3q21.1 (OMIM gene/locus number #601199).
- GNA11 gene, 19p13.3 (OMIM gene/locus number #139313).
- AP2S1 gene, 19q13.32 (OMIM gene/locus number #602242).
Phenotype
Usually asymptomatic, rare bone involvement, pancreatitis and chondrocalcinosis.
Main biochemical alterations
High Ca, low Ur Ca, CaCl/CrCl<0.01, high Mg, normal/high normal PTH.
They include developmental disorders with hypoparathyroidism as a common feature, in which cytogenetic alterations, such as microdeletion 22q11.2 responsible for DiGeorge syndrome, and mutations of PTH, GCM2 and CaSR have been excluded.
- Hypoparathyroidism, sensorineural deafness, renal disease (HDR, OMIM phenotype number #146255)
- Kenny-Caffey syndrome type 1-2 (KCS1, OMIM phenotype number #244460 ; KSC2, OMIM phenotype number #127000)
- Hypoparathyroidism, retardation, dysmorphism syndrome (HRD, OMIM phenotype number #241410)
- Gracile bone dysplasia (GCLEB, OMIM phenotype number #602361)
They include developmental disorders with hypoparathyroidism as a common feature, in which cytogenetic alterations, such as microdeletion 22q11.2 responsible for DiGeorge syndrome, and mutations of PTH, GCM2 and CaSR have been excluded.
They are transmitted as autosomal disorders mainly due to single gene heterozygous mutations and transmitted generally with a dominant pattern of inheritance with some exceptions. Hypoparathyroidism, usually manifesting early in life and due to parathyroid aplasia/hypoplasia, is associated to multiple organ morphological or functional abnormalities. Bone dysplasia may be the main feature.
In the syndrome of hypoparathyroidism, deafness and renal dysplasia (HDR, OMIM phenotype number #146255, also referred to as or Barakat syndrome, or nephrosis, nerve deafness and hypoparathyroidism) early onset hypoparathyroidism, usually manifesting within the first decade of life, is associated with mild-to-severe nephrosis due to renal dysplasia and congenital bilateral sensorineural deafness, with high variable phenotype. It is due to haploinsufficiency of GATA3 (OMIM gene number #131320 on chromosome 10p14), a transcription factor fundamental for the development of the parathyroids, kidney, inner ear, central nervous system, thymus and hematopoietic system. In the parathyroids GATA3 regulates the expression of GCM2, another key gene for parathyroid patterning and function. Mutations/deletions in DNA-binding domain and mutations in regions interacting with additional transcription factors are responsible for the HDR syndrome. No genotype-to-phenotype correlation exists. A specific bone phenotype has not been described, although feature of hypoparathyroidism-related bone disease may be present.
The main feature of Kenny-Caffey syndrome type 1 and 2 (KCS1, OMIM phenotype number #244460; KSC2, OMIM phenotype number #127000) is a bone dysplasia with severe postnatal growth retardation leading to dwarfism, long bones appear small and thin but with thickening of the cortex with medullary stenosis, osteosclerosis of the skull with macrocephaly, eye abnormalities, defective dentition associated with transient/recurrent or permanent hypoparathyroidism. KSC1 is due to mutations in the gene encoding tubulin-specific chaperone E (TBCE; OMIM gene number #604934 on chromosome 1q42.3) and follows a recessive pattern of inheritance, while KSC2 is due to heterozygous mutations in the gene FAM111A (OMIM gene number #615292 on chromosome 11q12.1) and follows an autosomal dominant pattern of inheritance.
The hypoparathyroidism, retardation and dysmorphism syndrome (HRD) (also referred to as Sanjad–Sakati syndrome, OMIM phenotype number #241410) is a disorder characterized by intrauterine growth retardation, mental retardation, congenital hypoparathyroidism with seizures, along with a typical facial dysmorphism (microcephaly, facial and dental anomalies, and small hands and feet), apparently lacking osteosclerosis and inherited in an autosomal recessive manner. It is due to mutations in the same gene whose alterations are responsible for KSC1 (TBCE; OMIM gene number #604934 on chromosome 1q42.3).
Gracile bone dysplasia (GCLEB, or osteocraniostenosis, OCS, OMIM phenotype number #602361) is a severe phenotype with high perinatal lethality in which hypoparathyroidism, developing in the few infants surviving the neonatal period, thin long bones with increased cortex causing medullary stenosis, microphtalmia, triangular face with frontal bossing, premature closure of basal cranial sutures are present. It is caused by mutations in the same gene responsible for KCS-2, namely FAM111A and follows an autosomal dominant pattern of inheritance.
Genes
- HDR: mutations in GATA3 (OMIM gene number #131320 on chromosome 10p14)
- KSC1: mutations in TBCE (OMIM gene number #604934 on chromosome 1q42.3)
- KSC2: mutations in FAM111A (OMIM gene number #615292 on chromosome 11q12.1)
- HRD: mutations in TBCE (OMIM gene number #604934 on chromosome 1q42.3)
- GCLEB: mutations in FAM111A (OMIM gene number #615292 on chromosome 11q12.1)
Phenotype
see above
Main biochemical alterations
Low Ca, high Pi, low/undetectable PTH, low 1,25(OH)2D; anemia and low magnesium can be present in KCS and GCLEB
Images
Figure: Phenotypic Features of KCS2 and GCLEB (Unger et al.)
Reproduced from Am J Hum Genet, Vol 92, Unger S, Górna MW, Le Béchec A, et al. FAM111A mutations result in hypoparathyroidism and impaired skeletal development, Pages 990-5, Copyright 2013, with permission from Elsevier.
Other resources:
Autoimmune polyendocrinopathy syndrome type 1 (APS1, also referred to as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome, APECED, OMIM phenotype number #240300) is a complex disorder usually inherited as an autosomal recessive trait, although an autosomal pattern of inheritance has been described in one kindred.
Types
Autoimmune hypoparathyroidism polyendocrine syndrome type 1 (APS1, OMIM phenotype number #240300)
Autoimmune hypoparathyroidism polyendocrine syndrome type 2 (APS2, OMIM phenotype number #269200)
Autoimmune polyendocrinopathy syndrome type 1 (APS1, also referred to as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy syndrome, APECED, OMIM phenotype number #240300) is a complex disorder usually inherited as an autosomal recessive trait, although an autosomal pattern of inheritance has been described in one kindred. The prevalence of APS1, usually referred to as a rare disease, can be relatively high in genetically isolated population because of a founder effect, i.e. 1:9000 in Iranian Jewish, 1:12000 in Sardinians and 1:2500 in Finnish. The clinical phenotype is characterized by the presence of the Whitaker’s triad, encompassing chronic mucoutaneous candidiasis, hypoparathyroidism and autoimmune adrenal insufficiency (i.e. Addison’s disease). Diagnosis of APS1 is made when at least two of these signs are present. Oral trush, representing a sing of T-cell deficiency, is generally present at birth, while hypoparathyroidism and Addison’s disease manifest later, usually in the first and third decades, respectively. Penetrance of hypoparathyroidism is high (60% in females and 100% in males). Other autoimmune and non-autoimmune diseases (such as autoimmune thyroid disease, vitiligo, insulin-dependent diabetes mellitus, primary gonadal failure, pernicious anemia, celiac disease, chronic active hepatitis, alopecia and other ectodermal anomalies) can be associated to the main manifestations, characterizing a various phenotype.
APS1 is cause by mutations in the autoimmune regulator type 1 gene (AIRE1, OMIM locus number #607358 on chromosome 21q22.3). More than 200 mutations have been identified, so far, including deletions, missense, non-sense and alternative splicing mutations. AIRE1 is expressed early in thymus, fetal spleen, liver, lymph nodes and peripheral hamtopoietic tissue. It is essential to initiate proper central and peripheral tolerogenic mechanisms, leading to multiorgan autoimmune alterations when altered or missing. A chronic inflammation with infiltration of autoreactive T-lymphocytes is present in involved glands/organs. Several non–specific autoantibodies can be detected in the serum, such as antibodies against T-helper type 17 cell-related cytokines type I interferon (IFN). Antibodies against parathyroid autoantigens, such as the ones against CaSR or NALP5, may be present but they cannot be used as a specific and sensitive marker of the diagnosis of hypoparathyroidism in APS1 or disease progression.
A specific APS1 bone phenotype has not been described, although features of long-standing hypoparathyroidism, such as increased trabecular and cortical thickness, in adult subjects can be present. In two cases heterosygous and homozygous for an AIRE1 13-bp deletion, a reversible metaphyseal dysplasia due to altered endochondral ossification has been described, characterized by skeletal deformities developing in childhood leading to short stature in childhood. Histologically, islands of calcified cartilagine within bone have been found. This abnormalities usually resolve at puberty.
Gene
Activating mutations of AIRE1 (OMIM locus number #607358 on chromosome 21q22.3).
Phenotype
Mucocutaneous candidiasis, and signs related to hypoparathyroidism and Addison’s disease.
Main biochemical alterations
APS1: hypocalcemia, low serum PTH levels, hyperphosphatemia, low serum 1,25(OH)2 vitamin D, hypocortisolism, high ACTH, defective response to ACTH in terms of cortisol; non-specific autoantibodies, anti-IFN1 antibodies, 21-Oh hydroxylase antibodies.
Images
Figure: features of metaphyseal dysplasia in two subjects with AIRE1 mutation and APS1
Reproduced from Am J Hum Genet, Vol 96, Rauch F, Fahiminiya S, Majewski J, et al. Cole-Carpenter syndrome is caused by a heterozygous missense mutation in P4HB, Pages 425-31, Copyright 2015, with permission from Elsevier.
Other resource:
DiGeorge Syndrome (OMIM phenotype number #188400) is the most frequent form of hypoparathyroidism associated with multiorgan impairment due to abnormalities in transcription factors, autoimmune dysregulation or mitochondrial alterations.
Type 1 (DGS1, OMIM phenotype number #188400)
Type 2 (DGS2, (OMIM phenotype number %601362)
DiGeorge Syndrome (OMIM phenotype number #188400) is the most frequent form of hypoparathyroidism associated with multiorgan impairment due to abnormalities in transcription factors, autoimmune dysregulation or mitochondrial alterations.
DiGeorge syndrome type 1 (DGS1, also referred to as chromosome 22q11.2 deletion syndrome or hypoplasia of thymus and parathyroids or third and fourth pharyngeal pouch syndrome) is the most frequent human disorder due to a DNA microdeletion. The birth prevalence is high, being 1:2000-4000 newborns. Since multiple organ abnormalities can be present in various combinations, the syndrome is also referred to as CATCH22, namely Cardiac defects, Abnormal facies, Thymic hypoplasia, Cleft palate, and Hypocalcaemia with microdeletion of chromosome 22. These developmental abnormalities involve the derivatives of the third and fourth parhyngeal pouches, such as the parathyroids and the thymus. It is characterized by a T-cell and a B-cell deficiency, craniofacial abnormalities (such as cleft palate, with other mouth, nose and eye alterations), anomalies of the heart and the great vessels and growth defects and hypoparathyroidism. Hypoparathyroidism can be present at birth, in the case of parathyroid aplasia, or manifest later in life if only hypoplasia occurs. It can be inherited in an autosomal dominant fashion or, more commonly, rather occur as a sporadic disease. Indeed, the 22q11.2 microdeletions causing DGS1 usually derive from meiotic non-allelic homologous recombination events between low-copy repeats. The deleted chromosomal region encompasses almost 60 genes comprising Tbx1. The absence of Tbx1, a transcription factor essential for embryonic patterning and development, is likely to play a key role in the pathogenesis of the syndrome. Indeed, in some cases of DGS negative for 22q11.2 microdeletions, loss-of-function mutations of Tbx1 have been detected. Cases of DGS negative for 22q11.2 mutations may display heterozygous deletion in chromosome 10p13-14, and therefore being referred to as DSG type 2 (DSG2, OMIM phenotype number %601362).
People of the disease show various grades of disease severity and combination of abnormalities and no genotype-to-phenotype correlation exists, although cases of late onset DGS1 tend to display similar cytogenetic defects. Interactions of Tbx1 with other factors coded by regions other than the 22q11.2 have been postulated to cause the wide variability of the phenotypes of DGS. Alternatively, it is possible that specific microRNA signatures or other environmental factors may modulate the expression of the genetic abnormality. Recently, a study in mice has demonstrated that the loss of Tbx1 induces a phenotype similar to cleidocranial dysplasia. No systematic assessment of bone in patients with DSG has been performed, yet.
Genes
Microdeletions in 22q11.2 region (DGS1) or 10p13-14 region (DGS2).
Phenotype
Thymic hypoplasia with T-cell deficiency, often early-onset, congenital hypocalcemia, with cardiac defects, craniofacial deformities, short stature.
Main biochemical alterations:
Neonates: low serum calcium; adults: low serum calcium in 65 % of cases, low serum PTH.
Images
Figure: two affected siblings from a family where DGS is inherited with an autosomal dominant pattern (Tbx1 loss-of-function mutation).
Reproduced from BMC Res Notes. 2014 Nov 5;7:783 under the terms of the Creative Commons Attribution 4.0 International (CC BY 4.0) License.
Other resources:
Mitochondrial diseases are a group of diseases caused by impairment of the respiratory chain. The prevalence is approximately 1–2/10.000. These disorders are subdivided in two groups: the disorders due to defects in mtDNA (mitochondrial DNA) and due to defects in nuclear DNA.
Kearns-Sayre syndrome (KSS) (OMIM phenotype number #530000)
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) (OMIM phenotype number #540000)
Mitochondrial trifunctional protein deficiency (MTPD) (OMIM phenotype number #609015)
Medium chain acylCoA dehydrogenase deficiency (ACADMD) (OMIM phenotype number #201450)
Mitochondrial diseases are a group of diseases caused by impairment of the respiratory chain. The prevalence is approximately 1–2/10.000. These disorders are subdivided in two groups: the disorders due to defects in mtDNA (mitochondrial DNA) and due to defects in nuclear DNA. MtDNA-related diseases are inherited according to the rules of mitochondrial genetics (maternal inheritance, heteroplasmy and the threshold effect, mitotic segregation), and mitochondrial diseases caused by abnormalities in nuclear DNA are inherited according to the Mendelian rules. The clinical features may be multisystem, involving visual and auditory pathways, heart, central nervous system, and skeletal muscle. The “red flags” are myopathy characterized by exercise intolerance, eyelid ptosis, ophthalmoparesis, axonal multifocal neuropathy, sensorineural hearing loss, pigmentary retinopathy, optic neuropathy, diabetes mellitus, hypertrophic cardiomyopathy, migraine-like headache, and short stature. Hypoparathyroidism is a very rare clinical manifestation among these diseases. Diagnosis of mitochondrial disease is based on biochemical exams, such as measurements of serum lactate at rest and after exercise, electromyography, muscle histology and enzymology, and molecular analysis. Serum creatine kinase concentrations are normal or moderately elevated. A specific and reliable biomarker is not available yet. In the most of cases, muscle biopsy is still necessary if there is a suspected mitochondrial disease. Mitochondrial hypoparathyroidism typically has a pediatric onset.
Kearns-Sayre syndrome (KSS) is a multisystemic disease defined by the following obligatory triad:
onset before age 20 years, pigmentary retinopathy, progressive external ophthalmoplegia. At least one of the following must also be present: cardiac conduction block, cerebrospinal fluid protein concentration greater than 100 mg/dL, and cerebellar ataxia. Other possible clinical features include short stature, hearing loss, dementia, limb weakness, diabetes mellitus, hypoparathyroidism, and growth hormone deficiency. Treatment of KSS is supportive.
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is a rare progressive multisystemic disease. First onset of symptoms is frequently between ages two and ten years (in some cases delayed onset between ages 10 and 40 years). The clinical diagnosis of MELAS is based on the following features: stroke-like episodes, typically before age 40 years, encephalopathy with seizures and/or dementia, and mitochondrial myopathy, evidenced by lactic acidosis and/or ragged red fibers on muscle biopsy. Two of the following are also required to confirm the diagnosis: normal early psychomotor development, recurrent headache, and recurrent vomiting. No specific treatment for MELAS exists.
Mitochondrial trifunctional protein deficiency (MTPD) is a very rare autosomal recessive disease of fatty acid oxidation characterized. This disorder is characterized by a wide heterogeneous clinical presentation with severe neonatal manifestations including cardiomyopathy, hypoglycemia, metabolic acidosis, skeletal myopathy and neuropathy, liver disease and death to a mild phenotype with peripheral polyneuropathy, episodic rhabdomyolysis and pigmentary retinopathy. There are two forms of MTPD, severe form (neonatal onset), and a moderately severe form (onset usually from the neonatal period to 18 months of age). Both forms can manifest with neuropathy with or without cardiomyopathy and can be fatal. Treatment management consists of a low fat diet with restriction of long chain fatty acid intake and substitution with medium chain fatty acids.
Medium-chain acyl-CoA dehydrogenase deficiency (MCADD) is an autosomal recessive disorder of mitochondrial fatty acid oxidation, often diagnosed in infancy or through neonatal screening. MCADD may be suspected in a previously healthy individual who becomes symptomatic with hypoketotic hypoglycemia, lethargy, seizures, coma triggered by a common illness, epatomegaly and acute liver disease. The first acute episode usually occurs before age two years, but affected individuals may present at any age including adulthood. The treatment in symptomatic patients is based on simple carbohydrates by mouth (e.g., glucose tablets, or sweetened, non-diet beverages) or IV if needed to reverse catabolism and sustain anabolism. The prevention of clinical manifestations is based on avoidance of fasting and weight control measures including proper nutrition and exercise.
Genes
KSS: gene, mitoc. DNA.
MELAS: gene, mitoc. DNA.
MTPD: HADHA gene, 2p23.3 (OMIM gene/locus number #600890); HADHB gene, 2p23.3 (OMIM gene/locus number #143450).
ACADMD: ACADM gene, 1p31.1 (OMIM gene/locus number #607008).
Phenotype
Severe multiorgan conditions, with high perinatal lethality, sometimes associated with hypoparathyroidism.
Main biochemical alterations
Low Ca, high Pi, low/undetectable PTH, low 1,25(OH)2D, high CSF proteins (>100 mg/dl), low CSF folic acid, lactic acidosis, low serum and muscle coenzyme Q. ACADMD: also low plasma carnitine levels.
Autosomal dominant hypocalcemia with hypercalciuria, also referred to as autosomal dominant hypercalciuric hypocalcemia or hypocalcemic hypercalciuria or familial hypocalcemia, is an inherited disorder of calcium metabolism, transmitted with a autosomal dominant pattern of inheritance.
Autosomal dominant hypocalcemia with hypercalciuria type 1 (HYPOC1)/Bartter syndrome subtype V (OMIM phenotype number #601198)
Autosomal dominant hypocalcemia with hypercalciuria type 2 (HYPOC2) (OMIM phenotype number and #615361)
Autosomal dominant hypocalcemia with hypercalciuria, also referred to as autosomal dominant hypercalciuric hypocalcemia or hypocalcemic hypercalciuria or familial hypocalcemia, is an inherited disorder of calcium metabolism, transmitted with a autosomal dominant pattern of inheritance. They belong to the group of isolated hypoparathyroidism, since no other alterations are present other than the abnormal parathyroid function.
In autosomal dominant hypocalcemia with hypercalciuria type 1 (HYPOC1), the abnormal regulation of PTH secretion is due to heterozygous mutations of calcium sensing receptor (CaSR, OMIM gene/locus OMIM number 601199 on chromosome 3q21), a membrane protein mainly expressed in the parathyroids and in renal tubule, which is capable of sensing small changes in extracellular calcium concentration [Cao2+]. When activated by small increases of the [Cao2+], the wild type CaSR triggers responses mediated by second messengers leading to an increase in intracellular calcium [Cai2+] fluxes, which ultimately inhibit PTH secretion and modulate renal mineral ion handling. Therefore, activating mutations of the CaSR result in a decrease of PTH, with consequent hypocalcemia with relative or absolute hypercalciuria. This is demonstrated by values of the urinary calcium creatinine (Ca/Cr) ratios within or above the upper reference limit.
Gain-of-function mutations of the CaSR are mostly missense (almost 100 identified, so far) and clustered in hot spots of the gene coding for critical conformational changes of the receptor. It is likely that the functional activity of the CaSR correlates with disease severity. HYPOC1 biochemical phenotype has been reproduced in the Nuf/+ or Nuf/Nuf mice, expressing one or two copies of CaSR, respectively, with the activating mutation Leu723Glu.
HYPOC1 is one of the most frequent causes of non-surgical, genetically determined hypoparathyroidism, with an estimated prevalence of less than 1:70000, with a F:M ratio of 1:1. No dysmorphic features are present. Hypocalcemia does not manifest at birth, but later in life, usually in the second-third decade. Hypocalcemia is mild or asymptomatic in 50% of cases, symptomatic in the other half of patients, with neuromuscular symptoms such as parestesias, but few episodes of carpo-pedal spasms, and seizures. More than one third of patients develop ectopic and basal ganglia calcifications. PTH concentrations are usually in the low-normal range. Serum magnesium levels are in the low-normal range, while serum phosphate is high. Bone films are usually negative. Bone mineral density as measured by DXA is normal or even increased. Nonetheless, few member of an identified kindred heterozygous for CaSR activating mutation, showed short stature and early osteoarthritis. Urinary excretion of calcium can be normal, but with relative hypercalciuria for the observed serum calcium levels, in the case of untreated mild disease, but it typically increases to overt hypercalciuria especially under treatment with active vitamin D analogs. Some patients may experience polyuria and polydipsia, because of the impaired water resorption mediated by enhanced CaSR-inhibition of vasopressin-dependent aquaporin-2-expression in the apical renal collecting duct. In very few cases, severe activating mutations of the CaSR cause a phenotype resembling the salt-wasting Bartter syndrome (also referred to as Bartter syndrome subtype V), characterized by hypocalcemia, hypomagnesemia, hypokalemia, metabolic alkalosis, hyperreninemia, and hyperaldosteronemia.
The recently identified autosomal dominant hypocalcemia with hypercalciuria type 2 (HYPOC2) is caused by heterozygous gain-of-function missense mutations of the guanine nucleotide binding protein (G Protein) Alpha 11 (GNA11). The altered protein increases the sensitivity to changes in [Cao2+].
No bone-signs specific for FIH exist. Nonetheless, increases in trabecular bone volume and cortical thickness have been reported, as in other forms of hypoparathyroidism.
In patients with autosomal dominant hypocalcemia, treatment with calcium and vitamin D analogs are likely to exhacerbate hypercalciuria, thus easily leading to nephrolithiasis and nephrocalcinosis. Therefore, it is advisable to treat only symptomatic patients, with the lowest dose of calcium and calcitriol. The addition of a thiazide diuretic may help in increasing serum calcium and lowering calciuria, thus decreasing the risk of nephrolithiasis. Also therapy with recombinant PTH has been demonstrated to lower the risk of renal complications. Future therapies might employ calcilytics, which directly modulate the CaSR.
Genes
Activating mutations of
Phenotype
Biochemical (see above in the main text); no radiological or physical signs, with the exception of signs of hypocalcemia in almost half of the cases.
Main biochemical alterations
Hypocalcemia, low or low-normal serum PTH levels, hyperphosphatemia, hypomagnesemia, high Ca/Cr ratio, low serum 1,25(OH)2 vitamin D.
REFERENCES
Bai M, Pearce SH, Kifor O, Trivedi S, Stauffer UG, Thakker RV, et al. In vivo and in vitro characterization of neonatal hyperparathyroidism resulting from a de novo, hetero- zygous mutation in the Ca2+-sensing receptor gene: normal maternal calcium ho- meostasis as a cause of secondary hyperparathyroidism in familial benign hypocalciuric hypercalcemia. J Clin Invest 1997;99:88–96.
Unger S, Górna MW, Le Béchec A, Do Vale-Pereira S, Bedeschi MF, Geiberger S, Grigelioniene G, Horemuzova E, Lalatta F, Lausch E, Magnani C, Nampoothiri S, Nishimura G, Petrella D, Rojas-Ringeling F, Utsunomiya A, Zabel B, Pradervand S, Harshman K, Campos-Xavier B, Bonafé L, Superti-Furga G, Stevenson B, Superti-Furga A. FAM111A mutations result in hypoparathyroidism and impaired skeletal development. Am J Hum Genet. 2013 Jun 6;92(6):990-5.
Harris, M., Kecha, O., Deal, C., Howlett, C. R., Deiss, D., Tobias, V., Simoneau-Roy, J., Walker, J. Reversible metaphyseal dysplasia, a novel bone phenotype, in two unrelated children with autoimmunepolyendocrinopathy-candidiasis-ectodermal dystrophy: clinical and molecular studies. J. Clin. Endocr. Metab. 88: 4576-4585, 2003.
Ogata T, Niihori T, Tanaka N, Kawai M, Nagashima T, Funayama R, Nakayama K, Nakashima S, Kato F, Fukami M, Aoki Y, Matsubara Y. TBX1 mutation identified by exome sequencing in a Japanese family with 22q11.2 deletion syndrome-like craniofacial features and hypocalcemia. PLoS One. 2014 Mar 17;9(3):e91598
Pearce S.H.S., Williamson C., Kifor O., Bai M., Coulthard M. G., Davies M., Lewis-Barned N., McCredie D., Powell H., Kendall-Taylor P., Brown E.M., Thakker R.V. A familial syndrome of hypocalcemia with hypercalciuria due to mutations in the calcium-sensing receptor. New Eng. J. Med. 335: 1115-1122, 1996.