DERANGED CALCIOTROPIC HORMONAL ACTIVITY
DISORDERS OF PHOSPHATE HOMEOSTASIS
Autosomal dominant hypophosphatemic rickets (ADHR) is a rare genetic disorder of phosphate homeostasis, caused by heterozygous point mutations at amino acid residues 176 or 179 in fibroblast growth factor 23 (FGF23).
(OMIM phenotype number #193100)
Autosomal dominant hypophosphatemic rickets (ADHR) is a rare genetic disorder of phosphate homeostasis, caused by heterozygous point mutations at amino acid residues 176 or 179 in fibroblast growth factor 23 (FGF23). These mutations disrupt enzymatic cleavage of the protein by a furin-like proprotein convertase, resulting in enhanced FGF23 bioactivity. FGF23, a secreted protein of 251 amino acids, reduces expression of sodium-phosphate co-transporters, NPT2a and NPT2c, on the apical surface of proximal renal tubule cells, resulting in renal phosphate wasting, and diminishs the renal 1α-hydroxylase and increases the 24-hydroxylase activity. Moreover, FGF23 acts at the parathyroid gland to decrease parathyroid hormone synthesis and secretion. FGF23, directly or indirectly, is involved in the pathogenesis of many diseases associated with low phosphate levels, such as tumor-induced osteomalacia (TIO), X-linked hypophosphatemic rickets (XLH), autosomal recessive hypophosphatemic rickets (ARHR), and McCune–Albright syndrome. ADHR is characterized by impaired mineralization of bone, rickets and/or osteomalacia, suppressed levels of calcitriol (1,25-dihydroxyvitamin D3), renal phosphate wasting, and low serum phosphate. This disease is the less common form of hypophosphatemic rickets, and has an incomplete penetrance and variable age of onset. Fluctuations in FGF23 concentration correlate with disease severity. Recent studies have revealed that iron could be one of the factors contributing to the regulation of FGF23 expression. Low iron levels were shown to correlate negatively with FGF23 levels in ADHR patients. In some cases remissions have been described.
Treatments for ADHR include: daily oral administration of phosphate and calcitriol, which prevents secondary hyperparathyroidism. Moreover, it is recommended a frequent monitoring of height, calcium, alkaline phosphatase, parathyroid hormone, and phosphate serum levels, urinary calcium and creatinine. In some cases, corrective surgery of skeletal deformities may be necessary.
Gene
FGF23 gene, 12p13.32 (OMIM gene/locus number #605380).
Phenotype
Fatigue and muscle weakness, short stature, early onset (1–3 years): severe bowing of lower extremities, rickets with enlarged costochondral junctions of the ribs; late onset (puberty): bone pain (no bowing of lower extremities).
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D; low-normal 1,25(OH)2D, high bone ALP, high intact FGF23, and normal PTH.
Fig. Imaging data of patient affecyed by ADHR. (a) X-ray of ankles and feet shows osteoporosis and two fractures of the second metatarsal bone of both feet (red arrows). (b) Bone scan shows multiple areas (cervical rib, vertebrae, articular genua, and ankle joints) of increased tracer uptake.
Reproduced from J Bone Miner Metab, FGF23 analysis of a Chinese family with autosomal dominant hypophosphatemic rickets, 2004;36:1213-8, Sun Y, Wang O, Xia W, et al., with permission of Springer.Vitamin D-dependent rickets type 2B (VDDR2B) is an unusual form of Vitamin D-dependent rickets due to abnormal expression of a hormone response element binding protein that interferes with the normal function of the VDR, without mutations in the VDR coding region.
(OMIM phenotype number #600785)
Vitamin D-dependent rickets type 2B (VDDR2B) is an unusual form of Vitamin D-dependent rickets due to abnormal expression of a hormone response element binding protein that interferes with the normal function of the VDR, without mutations in the VDR coding region. Hormone resistance resultes from constitutive overexpression of heterogeneous nuclear ribonucleoprotein (hnRNP) that competed with a normally functioning VDR-retinoid X receptor (RXR) dimer for binding to the vitamin D response element (VDRE). See also VDDR2A.
Phenotype
Growth retardation, muscle weakness, convulsion for hypocalcemia, bone pain at the lower extremities that delays their development of walking, dental caries or hypoplasia of the teeth, scalp and total alopecia, mild deafness, congenital rickets with fracture and pseudofractures, sparse bone trabeculae, thin bony cortex, delayed opacification of the epiphyses, widened, distorted epiphyses, frayed, irregular metaphyses, lower limb deformities, bowing of the legs, curvatures of the femur, tibia, fibula, enlargement of the wrists, enlargement of the ankles, and subperiosteal erosions due to secondary hyperparathyroidism.
Main biochemical alterations
High 1,25(OH)2D, markedly high bone ALP, low Ca and high or normal PTH.
Osteoglophonic dysplasia (OGD) is a very rare skeletal disease caused by activating mutations in a highly conserved domain in the gene encoding fibroblast growth factor receptor-1 (FGFR1). The name “osteoglophonic” was derived from the Greek word meaning a “hollowed-out” appearance of bone.
(OMIM phenotype number #166250)
Osteoglophonic dysplasia (OGD) is a very rare skeletal disease caused by activating mutations in a highly conserved domain in the gene encoding fibroblast growth factor receptor-1 (FGFR1). The name “osteoglophonic” was derived from the Greek word meaning a “hollowed-out” appearance of bone. Beighton called this disorder OGD in his review published in 1989. It is transmitted in an autosomal dominant manner. The FGFRs are part of a tyrosine kinase receptor family. They comprise an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular tyrosine kinase region. FGFR1 and FGFR2 mutations cause syndromes involving craniosynostosis whereas FGFR3 mutations are associated with dwarfing syndromes such as achondroplasia and hypochondroplastic dwarfism. OGD shares characteristics with both the craniosynostosis and dwarfing syndromes. The main radiographic features include: craniostenosis, fibrous dysplasia, metaphyseal lucencies and platyspondyly.
Gene
FGFR1 gene, 9p11.23-p11.22 (OMIM gene/locus number #136350).
Phenotype
Craniofacial abnormalities, macroglossia, hypertrophy of the gums, severe dwarfism, mandibular prognathism, frontal bossing, and proptosis; gross stunting of stature, rickets/osteomalacia, severe craniofacial abnormalities, and bone dysplasia.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D; low-normal 1,25(OH)2D, and high bone ALP, high/normal intact FGF23, normal PTH.
X-linked, dominant, hypophosphatemic rickets (XLHR) is a genetic disorder caused by inactivating mutations in the PHEX gene (phosphate-regulating gene with homologies to endopeptidase on the X chromosome). XLHR, first described by Albright in 1937, is the most frequent form of hypophosphatemic rickets, with a prevalence of 1/20.000. The disease affects both sexes.
(OMIM phenotype number #307800)
X-linked, dominant, hypophosphatemic rickets (XLHR) is a genetic disorder caused by inactivating mutations in the PHEX gene (phosphate-regulating gene with homologies to endopeptidase on the X chromosome). XLHR, first described by Albright in 1937, is the most frequent form of hypophosphatemic rickets, with a prevalence of 1/20.000. The disease affects both sexes. Mutations of PHEX inhibit FGF23 inactivation, and the latter has been reported to be 5 times higher in XLH patients compared to healthy controls. High free FGF23 levels in plasma cause hyperphosphaturia, decreasing phosphate reabsorption by down-regulating NaPi-IIa and NaPi-IIc at the apical membrane of proximal renal tubular cells and inhibit 1a-hydroxylase, resulting in decreased intestinal absorption of calcium and phosphate (see also ADHR). Chronic low serum phosphorus levels lead to defective bone mineralization and, consequently, to rickets in children and osteomalacia in adults. During the first 1–2 years of life, children with XLHR may present bowing of the weight-bearing extremities, frontal bossing, and growth retardation. Others clinical manifestations, such as joint pain, arthritis, enthesopathy (calcification of ligaments and their attachment to bone), dentin malformation, and recurrent spontaneous abscesses of the teeth may occur with increasing age. Radiographic features include: genu varum, widened metaphyses, bilateral femoral distal metaphysis epiphyseal line blurred, and a general decrease in bone density. Molecular genetic testing confirms the diagnosis.
Treatment: For most children consists of oral phosphate and high-dose calcitriol, the active form of vitamin D. In adults, treatment with calcitriol and phosphate is generally reserved in the case of symptoms, such as bone pain, upcoming orthopedic surgery, biochemical evidence of osteomalacia with an elevated alkaline phosphatase, or recurrent pseudofractures or stress fractures. Currently, a novel therapeutic agent KRN23 is under investigation for XLH. KRN23 is a recombinant human IgG1 monoclonal antibody that binds to FGF23 and blocks its activity.
Gene
PHEX gene, Xp22.11 (OMIM gene/locus number #300550).
Phenotype
Rickets, osteomalacia, late dentition, tooth abscesses secondary to poor mineralization, bowing of lower extremities, enlarged costochondral junctions of the ribs, pectum carinatum, metaphyseal flaring of the wrists or ankles, genus varus, frontal bossing enlarges sutures and fotanels or craniotabes.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D, low-normal 1,25(OH)2D, high bone ALP, high intact FGF23, normal PTH, and rarely low GH.
Images
Fig. Patients affected by XLHR. (a,b) Radiographs and (c) image showing genu varus deformities in the lower extremities. (d) Patient with dental crowding and dental absences.
Reproduced from Gene, Vol 565, Huang Y, Mei L, Pan Q, et al. Novel de novo nonsense mutation of the PHEX gene (p.Lys50Ter) in a Chinese patient with hypophosphatemic rickets, Pages 150-4, Copyright 2015, with permission from ElsevierOther resources:
Autosomal recessive hypophosphatemic rickets (ARHR) is an extremely rare disease, characterized by hypophosphatemia resulting from renal phosphate wasting (See also ARHR1).
(OMIM phenotype number #613312)
Autosomal recessive hypophosphatemic rickets (ARHR) is an extremely rare disease, characterized by hypophosphatemia resulting from renal phosphate wasting (See also ARHR1). Autosomal recessive hypophosphatemic rickets type 2 (ARHR2) is caused by homozygous loss-of-function mutation in the ENPP1 gene. ENPP1 encodes a protein called ectonucleotide pyrophosphatase/ phosphodiesterase 1 (NPP1), which is a major generator of extracellular pyrophosphate (PPi). Because PPi inhibits calcification (hydroxyapatite crystal deposition and growth), homozygous inactivating mutations in ENPP1 gene are also responsible for generalized arterial calcification of infancy (see also GACI). In patients with ARHR2, high circulating levels of FGF23 have been described. FGF23 is a secreted protein, which reduces expression of sodium-phosphate co-transporters, NPT2a and NPT2c, resulting in renal phosphate wasting, diminishs the renal 1α-hydroxylase and increases the 24-hydroxylase activity. Moreover, FGF23 acts at the parathyroid gland to decrease parathyroid hormone synthesis and secretion. Currently, it is unclear how mutations in ENPP1 gene results in high FGF23 levels.
Gene
ENPP1 gene, 6q23.2 (OMIM gene/locus number *173335)
Phenotype
Short stature; limited movement of spine and hip, calcification of the ligaments at the bony insertions sites, high bone density at the base of skull, clavicle and rib anomalies, and enthesopathies.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D; low-normal 1,25(OH)2D, high bone ALP, high intact FGF23, and normal PTH.
Image
Fig. Radiography shows dense ossification of the anterior longitudinal ligament.
Reproduced from Mehta P, Mitchell A, Tysoe C, et al., Novel compound heterozygous mutations in ENPP1 cause hypophosphataemic rickets with anterior spinal ligament ossification, Rheumatology (Oxford) 2012;51:1919-21, by permission of Oxford University Press.
Other resources:
Hypoparathyroidism is an uncommon endocrine-deficiency disorder characterized by low serum calcium levels, elevated serum phosphorus levels, and absent or inappropriately low levels of parathyroid hormone (PTH).
(OMIM phenotype number #241520)
Autosomal recessive hypophosphatemic rickets (ARHR) is an extremely rare disease, characterized by hypophosphatemia resulting from renal phosphate wasting. It has been described in few families of Middle Eastern and European origins. Clinical manifestations include: growth retardation, lower-extremity deformities, pathologic fractures, dental defects, and, in some patients, also enthesopathy. Patients also show hypophosphatemia, low levels of serum 1,25-dihydroxyvitamin D, whereas serum calcium, parathyroid hormone, urinary calcium excretion are normal, and high circulating levels of FGF23. ARHR is subdivided in two subtypes: Autosomal recessive hypophosphatemic rickets type 1 (ARHR1) and type 2 (ARHR2). ARHR1 is caused by homozygous loss-of-function mutations in the DMP1 (Dentin matrix protein 1) gene. DMP1 is a noncollagenous extracellular protein, highly expressed in osteoblasts and osteocytes, in bone and teeth. It plays critical roles in bone mineralization, phosphate homeostasis and odontogenic differentiation.
Gene
DMP1 gene, 4q22.1 (OMIM gene/locus number #600980)
Phenotype
Short stature; limited movement of spine and hip, calcification of the ligaments at the bony insertions sites, high bone density at the base of skull, clavicle and rib anomalies, enthesopathies.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D; low-normal 1,25(OH)2D, high bone ALP, high intact FGF23, and normal PTH.
Fig. Skeletal and dental findings in patient affected by ARHR1, at the age of 53 years. (a, b) Radiographs show mild bowing of her legs and severe kyphosis. (c) A panoramic radiograph shows loss of all her teeth.
Reproduced from J Bone Miner Metab, A novel nonsense mutation in the DMP1 gene in a Japanese family with autosomal recessive hypophosphatemic rickets, 2010;28:585-90, Koshida R, Yamaguchi H, Yamasaki K, et al., with permission of Springer.Hypophosphatemic rickets with hyperparathyroidism (HRH) is a form of hypophosphatemic rickets with marked parathyroid hyperplasia. It has been demonstrate that HRH is caused by a mutation that results in increased levels of circulating α-Klotho. It has been described a de novo translocation with a breakpoint adjacent to α-Klotho.
(OMIM phenotype number %612089)
Hypophosphatemic rickets with hyperparathyroidism (HRH) is a form of hypophosphatemic rickets with marked parathyroid hyperplasia. It has been demonstrate that HRH is caused by a mutation that results in increased levels of circulating α-Klotho. It has been described a de novo translocation with a breakpoint adjacent to α-Klotho. It encodes a α-glucuronidase, and is implicated in aging and regulation of FGF (Fibroblast Growth Factor) signaling. High plasma α-Klotho levels and α- glucuronidase activity have been described in HRH, and unexpectedly, also high circulating FGF23 level. These findings suggest that the elevated α-Klotho concentration mimics aspects of the normal response to hyperphosphatemia. When phosphate is in excess, FGF23 is secreted from bone and acts on the kidney to promote phosphate excretion into urine and suppress vitamin D synthesis (see also XLHR). Klotho, a single-pass transmembrane protein expressed in renal tubules, is an obligate coreceptor to bind and activate FGF receptors isoforms (FGFR1c, 3c, 4) and significantly increases the affinity of these FGFRs specifically to FGF23.
Gene
KLOTHO gene, 13q13.1 (OMIM gene/locus number 604824).
Phenotype
Kidney stones, rickets, bone pain, and bowing of lower extremities.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal Ca, low-normal Ur Ca, normal 25 OH D; low-normal 1,25(OH)2D, high bone ALP, high PTH (normal intact FGF23).
Generalized arterial calcification of infancy (GACI) is caused, in most cases, by homozygous or compound heterozygous inactivating mutation in the ENPP1 gene, resulting in reduced plasma inorganic pyrophosphate levels.
(OMIM phenotype number #208000)
Generalized arterial calcification of infancy (GACI) is caused, in most cases, by homozygous or compound heterozygous inactivating mutation in the ENPP1 gene, resulting in reduced plasma inorganic pyrophosphate levels. It is an autosomal recessive disorder. GACI is characterized by early mineralization of blood vessels, but also on other soft connective tissues, often diagnosed by prenatal ultrasound. In most cases patients affected by GACI die during the first six months of life from cardiovascular complications. ENPP1 gene, encodes an enzyme that hydrolyzes ATP to AMP and inorganic pyrophosphate (PPi), the latter being a powerful anti-mineralization factor. The loss-of-function mutations in the ENPP1 gene results in decreased synthesis of PPi, causing a low PPi/Pi ratio which then allows the ectopic mineralization processes to ensue. Loss-of-function ENPP1 mutations can also cause autosomal recessive hypophosphatemic rickets type 2 (see also ARHR2).
Treatment: There is no effective or specific treatment for GACI. Recently, a study on animal model has described that bisphosphonate treatment may be beneficial by a dual effect for preventing ectopic soft tissue mineralization while correcting decreased bone mineralization in GACI caused by ENPP1 mutations.
Gene
ENPP1 gene, 6q23.2 (OMIM gene/locus number #173335).
Phenotype
Short stature, deafness, conductive (in some patients), angioid retinal streaks (in some patients), coronary and generalized artery calcification, cardiac dysfunction, periarticular calcification, hypophosphatemic rickets, and pseudoxanthoma.
Main biochemical alterations
Low Pi.
Images
Fig. (a) Lateral radiograph of the neonate showing calcification of descending aorta and its bifurcation (arrows). (b) Ultrasonography of abdominal aorta showing calcification of descending aorta and its branches (arrows).
Reproduced from J Pediatr, Vol 162, Karthikeyan G, Generalized arterial calcification of infancy, Pages 1074.e1., Copyright 2013, with permission from Elsevier.Hypophosphatemia nephrolithiasis osteoporosis type 1 (NPHLOP1) is caused by heterozygous mutation in the SLC34A1 gene. The gene encodes the protein for the type 2a sodium–phosphate cotransporter (NPT2a), which resides in the apical membrane of renal proximal tubular cells.
(OMIM phenotype number #612286)
Hypophosphatemia nephrolithiasis osteoporosis type 1 (NPHLOP1) is caused by heterozygous mutation in the SLC34A1 gene. The gene encodes the protein for the type 2a sodium–phosphate cotransporter (NPT2a), which resides in the apical membrane of renal proximal tubular cells. Phosphate homeostasis is principally regulated by the kidney, modulating urinary phosphate excretion. SLC34A1 mutations have been identified in few patients with impairment in renal phosphate reabsorption, resulting in hypophosphatemia, associated with urolithiasis or bone demineralization of variable severity in both sexes.
Gene
SLC34A1 (NPT2a) gene, 5q35.3 (OMIM gene/locus number #182309).
Phenotype
Kidney stones, nephocalcinosis, osteoporosis.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal calcium, high Ur Ca, low/normal PTH, normal 25 OH D, high 1,25(OH)2D, high bone ALP, normal Ca, and normal intact FGF23.
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a rare autosomal recessive disorder, caused by homozygous or compound heterozygous mutation in the SLC34A3 gene. SLC34A3 gene encodes the sodium (Na+)-dependent phosphate cotransporter 2c (NaPi-IIc). NaPi-IIc, with NaPi-IIa encoded by SLC34A1, reabsorb most of filtered phosphate in the renal proximal tubule.
(OMIM phenotype number #241530)
Hereditary hypophosphatemic rickets with hypercalciuria (HHRH) is a rare autosomal recessive disorder, caused by homozygous or compound heterozygous mutation in the SLC34A3 gene. SLC34A3 gene encodes the sodium (Na+)-dependent phosphate cotransporter 2c (NaPi-IIc). NaPi-IIc, with NaPi-IIa encoded by SLC34A1, reabsorb most of filtered phosphate in the renal proximal tubule. The expression of these two co-transporters are regulated by two phosphaturic hormones, such as PTH and FGF23. HHRH is characterized by renal phosphate wasting resulting in hypophosphatemia, correspondingly elevated 1,25(OH)2 vitamin D levels, hypercalciuria, rickets/osteomalacia, short stature, bone pain, and muscle weakness. The hypercalciuria can lead to the formations of kidney stones and/or the occurrence of nephrocalcinosis. This disease requires phosphate supplements alone, without calcitriol supplementation as this may increase 1,25(OH)2 vitamin D levels resulting to hyperabsorptive hypercalciuria. HHRH is distinguished from XLH and ADHR by the presence of an appropriately elevated level of serum 1,25(OH)2 vitamin D levels concentration, leading to hypercalciuria and suppression of PTH secretion.
Gene
SLC34A3 (NPTIIc) gene, 9q34.3 (OMIM gene/locus number #609826).
Phenotype
Kidney stone, nephrocalcinosis, rickets/osteomalacia, growth retardation, frontal bossing, increased fractures, bone pain, hypotonia, muscle weakness, difficulty walking, and difficulty standing.
Main biochemical alterations: High Ur P, low Pi, low renal TmP/GFR, normal calcium, high Ur Ca, low/normal PTH, normal 25 OH D, high 1,25(OH)2D, and high bone ALP, normal/high Ca, and normal intact FGF23.
Images
Fig. Radiographies of a patient affected by HHRH. (A) X-ray of the hip and femur shows bilateral long bone bowing and general osteomalacia. (B) Renal ultrasound shows multiple stones in the right kidney. (C) Bone scan shows multiple areas (ribs, hips, knees, skull) of increased tracer uptake and tracer retention in the renal calices.
Reproduced from Bone, Vol 59, Chi Y, Zhao Z, He X et al. A compound heterozygous mutation in SLC34A3 causes hereditary hypophosphatemic rickets with hypercalciuria in a Chinese patient, Pages 114-21, Copyright 2014, with permission from Elsevier.
Familial tumoral calcinosis (FTC) is a rare disease characterized by hyperphosphatemia due to hypophosphaturia and by ectopic calcifications, in cutaneous and subcutaneous tissues, especially around large joints (hip, elbow or shoulder).
(OMIM phenotype number #211900)
Familial tumoral calcinosis (FTC) is a rare disease characterized by hyperphosphatemia due to hypophosphaturia and by ectopic calcifications, in cutaneous and subcutaneous tissues, especially around large joints (hip, elbow or shoulder). Calcifications lead to painful skin ulcerations, secondary skin and bone infections, and contractures. FTC is inherited in an autosomal recessive mode, but autosomal dominant inheritance has also been described. The disease usually appears before the second decade of life. Two forms of FTC have been described: Hyperphosphatemic familial tumoral calcinosis (HFTC), and Normophosphatemic FTC (NFTC). HFTC, also called hyperostosis hyperphosphatemia syndrome (HHS), is caused by mutations in the GALNT3, FGF23, and KLOTHO gene. It is a rare congenital disorder in which the differential diagnosis from other syndromes associated with extraskeletal calcifications may be difficult. The disease was initially found to result from mutations in GALNT3 encoding a glycosyltransferase, and mutations in FGF23, encoding a potent phosphaturic protein. Recent studies have shown that FGF23 requires an additional co-factor, Klotho (KL), to bind and signal through its cognate fibroblast growth factor receptors (FGFRs), and loss-of-function mutations in human KL impair FGF23 bioactivity, leading to severe tumoral calcinosis. HFTC represents the metabolic mirror image of autosomal dominant hypophosphatemic rickets (ADHR) caused by gain of function mutations in the fibroblast growth factor 23 (FGF23) gene.
Treatment of tumoral calcinosis varies depending on the type of the lesion, stage of the pathology, and symptoms of the patient. Primary treatment is early surgical excision, although there is a high rate of recurrence. Medical therapies with phosphate depletion, dietary deprivation of phosphorus and phosphate binding chelating agents, such as oral aluminium hydroxide, has shown variable positive outcomes in both NFTC and HFTC.
Gene
- FGF23 gene, 12p13.32 (OMIM gene/locus number #605380)
- GALNT3 gene, 2q24.3 (OMIM gene/locus number #601756)
- KLOTHO gene, 13q13.1 (OMIM gene/locus number #604824)
Phenotype
Periarticular cystic and solid tumoral calcifications with hyperphosphatemia and hypophosphaturia and elevated serum of calcitriol, soft tissue masses around major joints, dental abnormalities, ocular involvement with range from angioid streaks to corneal calcification deposits and neuronal calcifications, altered skeletal mineralization, and low/normal bone mass.
Main biochemical alterations
Low Ur P, high Pi, high TmP/GFR, normal Ca, normal Ur Ca, normal PTH, normal 25 OH D, high 1,25(OH)2D, and low intact FGF23.
Hypophosphatemia nephrolithiasis osteoporosis type 2 (NPHLOP2) is caused by heterozygous mutation in the SLC9A3R1 or NHERF1 gene. NHERF1, the sodium-hydrogen exchanger regulatory factor 1, binds to the main renal phosphate transporter NPT2a and to the parathyroid hormone (PTH) receptor.
(OMIM phenotype number #612287)
Hypophosphatemia nephrolithiasis osteoporosis type 2 (NPHLOP2) is caused by heterozygous mutation in the SLC9A3R1 or NHERF1 gene. NHERF1, the sodium-hydrogen exchanger regulatory factor 1, binds to the main renal phosphate transporter NPT2a and to the parathyroid hormone (PTH) receptor. NHERF1 mutations cause decreased renal phosphate reabsorption by increasing PTH-induced cAMP production in the renal proximal tubule. Impaired renal phosphate reabsorption (measured by dividing the tubular maximal reabsorption of phosphate by the glomerular filtration rate (TmP/GFR)), increases the risks of nephrolithiasis and bone demineralization.
Gene
SLC9A3R1 gene, 17q25.1 (OMIM gene/locus number #604990)
Phenotype
Kidney stones, nephocalcinosis, osteoporosis.
Main biochemical alterations
High Ur P, low Pi, low renal TmP/GFR, normal calcium, high Ur Ca, low/normal PTH, normal 25 OH D, high 1,25(OH)2D, high bone ALP, normal Ca, and normal intact FGF23.
Normophosphatemic familial tumoral calcinosis (NFTC) is an autosomal recessive disorder characterized by calcium deposition in skin and mucosae, unremitting pain and life-threatening skin infections. It can be caused by mutation in the gene encoding the sterile alpha motif domain-containing-9 protein (SAMD9).
(OMIM phenotype number #610455)
Normophosphatemic familial tumoral calcinosis (NFTC) is an autosomal recessive disorder characterized by calcium deposition in skin and mucosae, unremitting pain and life-threatening skin infections. It can be caused by mutation in the gene encoding the sterile alpha motif domain-containing-9 protein (SAMD9). Calcifications usually develop over areas subjected to repeated trauma and are associated with marked inflammatory manifestations. It has been hypothesized that SAMD9 may be under the regulation of TNF-α (a major pro-inflammatory cytokine and inducer of apoptosis), probably, involved in the pathogenesis of extra-osseous calcification. Recently, it has been suggested that SAMD9 is an interferon- γ (IFN-γ)-responsive protein, and interacts with RGL2, diminishing the expression of EGR1, a important protein for the pathogenesis of ectopic calcification and inflammation. At last, several studies have suggested that SAMD9 might have also a role as a tumor suppressor gene, but no increased incidence of cancerous conditions has been described in this disease. However, further studies are needed to elucidate the physiological function of SAMD9.
Gene
SAMD9 gene, 7q21.2 (OMIM gene/locus number #610456)
Phenotype
Reddish to hyperpigmented skin lesions, soft tissue masses at the extremities, calcified ulcerating nodules (massive calcium deposition in mid- and lower dermis), severe conjunctivitis, severe gingivitis, and no altered skeletal mineralization.
Main biochemical alterations
Normal Pi, and normal Ur P.
Images
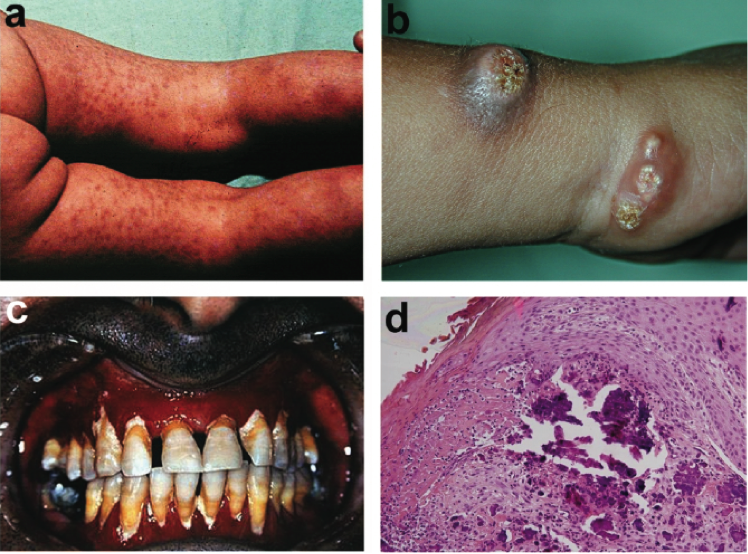
Fig. Clinical features of NFTC, including heralding erythematous papular eruption (a), small calcified tumors ulcerating and discharging chalk-like materials on the surface of the skin (b), and severe gingivitis (c) and calcified material in the upper dermis, as revealed by histopathological examination (d).
Reproduced from Am J Hum Genet, Vol 79, Topaz O, Indelman M, Chefetz I, et al. A deleterious mutation in SAMD9 causes normophosphatemic familial tumoral calcinosis, Pages 759-64, Copyright 2006, with permission from Elsevier.REFERENCES
Ferreira C, Ziegler S, Gahl W. Generalized Arterial Calcification of Infancy. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, Bird TD, Fong CT, Mefford HC, Smith RJH, Stephens K, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. 2014 Nov 13.
Li Q, Kingman J, Sundberg JP et al. Dual Effects of Bisphosphonates on Ectopic Skin and Vascular Soft Tissue Mineralization versus Bone Microarchitecture in a Mouse Model of Generalized Arterial Calcification of Infancy. J Invest Dermatol. 2015 Sep 29. doi: 10.1038/jid.2015.377. [Epub ahead of print]