ALTERED OSTEOCLAST, OSTEOBLAST OR OSTEOCYTE ACTIVITY
High Bone Formation
Sclerosteosis is a severe autosomal-recessive sclerosing bone dysplasia, characterized by progressive skeletal overgrowth, distortion of the facies, and entrapment of cranial nerves (see also VBD).
(OMIM phenotype number #269500)
Sclerosteosis is a severe autosomal-recessive sclerosing bone dysplasia, characterized by progressive skeletal overgrowth, distortion of the facies, and entrapment of cranial nerves (see also VBD). Sclerosteosis can be distinguished from Van Buchem disease by the presence of syndactyly and a tendency to tall stature. The jaw has an unusually square appearance in this condition. Potentially lethal elevation of intracranial pressure is a frequent complication which has been recognized in children as young as 5 years of age. This disorder is rare and occurs primarily in Afrikaners (South Africa) or others of Dutch ancestry.
Gene
SOST gene, 17q21.31 (OMIM gene/locus number #605740)
Phenotype
Symmetric cutaneous syndactyly, excessive height and weight, frontal prominence, midface hypoplasia, vision loss, optic atrophy, deafness, facial palsy, cranial hyperostosis (onset in infancy), occlusion of cranial foramina, hypertelorism, proptosis, convergent strabismus, nystagmus, broad and flat nasal root, prognathism, dental malocclusion, prominent and asymmetric mandible, chronic headaches, intellectual impairment, increased intracranial pressure, nail dysplasia, broad and dense clavicles-ribs, sclerotic scapulae, sclerotic vertebral endplates and pedicles, sclerotic pelvis, cortically dense long tubular bones, and lack of diaphyseal modeling.
Focal dermal hypoplasia, or Goltz-Gorlin syndrome, is a rare syndrome, which may affect many different organs such as eyes, bones (limb malformation, osteopathia striata, costovertebral abnormalities, slipt sternum, fibrous dysplasia of bone, syndactyly, polydactyly, “lobster-clawlike” oligodactyly, short stature), teeth, and skin. To date, more than 280 cases of FDH have been reported in the literature.
(OMIM #305600)
Focal dermal hypoplasia, or Goltz-Gorlin syndrome, is a rare syndrome, which may affect many different organs such as eyes, bones (limb malformation, osteopathia striata, costovertebral abnormalities, slipt sternum, fibrous dysplasia of bone, syndactyly, polydactyly, “lobster-clawlike” oligodactyly, short stature), teeth, and skin. To date, more than 280 cases of FDH have been reported in the literature. Clinical findings vary from mild skin atrophy to severe limb deformity and life-threatening complications. This disorder is inherited in an X-linked dominant manner, and occurs in females (90%). Approximately 95% of reported female cases occur sporadically as a result of de novo mutations. Mutation of PORCN (porcupine homolog) gene is closely associated with this disease. PORCN, located at Xp11.23, encodes the human homolog of Drosophila melanogaster Porcupine. Although the biochemical functions of the PORCN gene have not been completely characterized, it is known that Porcupine proteins are involved in the processing of Wnt (wingless and int homologue) proteins. Wnt signaling has been implicated in the embryonic development of the skin, bone, and other structures.
The goal of treatment is to improve specific system function and cosmetic appearance. Surgical interventions are often needed during childhood for the correction of skin, skeletal, intestinal, and ophthalmologic alterations. Therapeutic management includes: reconstructive surgery, and orthopedic surgery. Basic treatment for skin abnormalities is to promote healing and avoid infection. The flashlamp-pumped pulse dye laser has been reported to reduce pruritus and erythema and can flatten lesions. It has also been described that recalcitrant excessive granulation tissue could be treated with photodynamic therapy.
Gene
PORCN gene, Xp11.23 (OMIM gene/locus number #300651)
Phenotype
Short stature, mild microcephaly, facial asymmetry, pointed chin, protruding, simple and low-set ears, narrow auditory canals, hearing loss, mixed, strabismus, iris coloboma, aniridia, microphthalmia (15%), anophthalmia, choroidoretinal coloboma, ectopia lentis (6%), aniridia (3%), optic atrophy, nystagmus, decreased visual acuity, narrow nasal bridge, broad nasal tip, notched nasal alae, papillomas (lip and gingiva), cleft lip, cleft palate, hypodontia, oligodontia, enamel hypoplasia, delayed eruption, malocclusion, notched incisors, papillomatosis, midclavicular aplasia, midclavicular hypoplasia, rib hypoplasia, supernumerary nipples, symmetric breast, nipple hypoplasia, diaphragmatic hernia, umbilical hernia, omphalocele, diastasis recti, hiatus hernia, anteriorly displaced anus, intestinal malrotation, esophageal papillomas, inguinal hernia, clitoral hypoplasia, labial hypoplasia, cryptorchidism, horseshoe kidney, hydronephrosis, bifid ureter, skeletal asymmetry, joint laxity, asymmetric skull, failure of pubic bone fusion, congenital hip dislocation, osteopathia striata, syndactyly (75%), brachydactyly (60%), oligodactyly (45%), ectrodactyly, postaxial polydactyly, short phalanges, short metacarpal, short metatarsal, syndactyly, hypoplastic digits, missing toes, ectrodactyly, polydactyly, linear or reticular hyperpigmentation, skin atrophy, telangiectasia, localized cutaneous deposits of superficial fat, arborescent papillomas (axillae, periumbilical area, anus, vulva), hypoplastic fingertip epidermal ridges, hidrocystomas, dystrophic nails (spooned, grooves), absent fingernails, absent toenails, sparse hair, brittle hair, patchy alopecia (head, pubic area), mental retardation (15%), myelomeningocele, hydrocephalus, agenesis of corpus callosum, Arnold-Chiari malformation.
Images
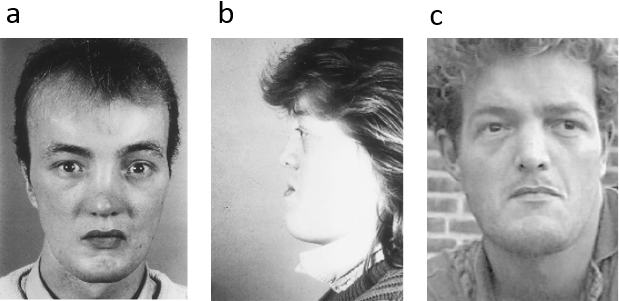
Fig. (a) Syndactyly with lobster claw deformity of the left hand. (b) Oligodactyly and syndactyly of the left foot.
Reproduced from Tenkir A, Teshome S, Goltz syndrome (focal dermal hypoplasia) with unilateral ocular, cutaneous and skeletal features: case report. BMC Ophthalmol 2010;10:28.
Other resources
Van Buchem disease type 2, autosomal dominant, is sclerosing bone dysplasia, that can be caused by mutation in the Low-Density Lipoprotein Receptor-Related Protein-5 (LRP5) gene (see also VBD).
(OMIM #607636)
Van Buchem disease type 2, autosomal dominant, is sclerosing bone dysplasia, that can be caused by mutation in the Low-Density Lipoprotein Receptor-Related Protein-5 (LRP5) gene (see also VBD).
Gene
LRP5 gene, 11q13.2 (OMIM gene/locus number *603506)
Phenotype
Increased bone density, mostly asymptomatic, associated with osteosclerosis of the skull, increased calvarial thickness, enlarged and squared jaw (decreased gonial angle), enlarged mandible, cranial nerve compression, sensorineural hearing loss (otopharyngeal exostosis), and thickened cortices of long bones.
Sclerosteosis 2, autosomal dominant/recessive, is a sclerosing bone dysplasia caused by heterozygous or homozygous mutation in the Low density lipoprotein receptor related protein 4 (LRP4) gene (see also VBD). It has been described that the interaction of sclerostin with LRP4 is required to mediate the inhibitory function of sclerostin on bone formation (see also SOST1).
(OMIM #614305)
Sclerosteosis 2, autosomal dominant/recessive, is a sclerosing bone dysplasia caused by heterozygous or homozygous mutation in the Low density lipoprotein receptor related protein 4 (LRP4) gene (see also VBD). It has been described that the interaction of sclerostin with LRP4 is required to mediate the inhibitory function of sclerostin on bone formation (see also SOST1).
Gene
LRP4 gene, 11p11.2 (OMIM gene/locus number *604270)
Phenotype
Syndactyly/brachyphalangy with nail dysplasia, increased stature, increased head circumference, facial asymmetry due to facial nerve palsy, frontal bossing, prognathism, hearing loss, hypertelorism, wide and dense clavicles-ribs, sclerotic calvarium, enlarged and sclerotic mandible, sclerotic vertebral end plates and pedicles, sclerotic pelvic bones, cortical hyperostosis of long bones, radial deviation of distal phalanx, facial nerve palsy, and spastic-ataxic tetraparesis.
Images
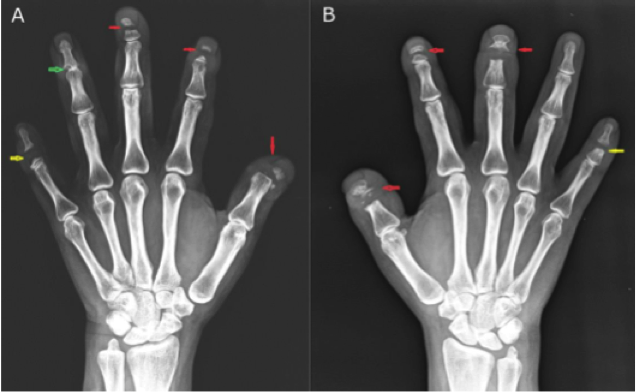
Fig. (a) Radiographs of the hands show modeling defect of the metacarpal bones and complex fusion anomaly of the individual bones of the hands. (b) Radiograph of the skull (lateral view) shows extensive sclerosis of the calvaria, maxilla, mandible, and cervical spine. (c) Radiograph of the left lower shows thickening of the cortical bone of the diaphysis of the tibia with extension into the proximal metaphysis.
This research was originally published in J Biol Chem. Leupin O, Piters E, Halleux C, et al. Bone overgrowth-associated mutations in the LRP4 gene impair sclerostin facilitator function. J Biol Chem. 2011; 286:19489-500. © The American Society for Biochemistry and Molecular Biology
Endosteal hyperostis include different uncommon disorders are included in this category, such as: the autosomal recessive conditions (Hyperostosis corticalis generalisata/Van Buchem disease), as well as the autosomal dominant conditions (Van Buchem disease type 2, Osteosclerosis/endosteal hyperostosis), and the autosomal dominant - recessive conditions (Sclerosteosis 1-2).
(OMIM phenotype number #239100)
Endosteal hyperostis include different uncommon disorders are included in this category, such as: the autosomal recessive conditions (Hyperostosis corticalis generalisata/Van Buchem disease), as well as the autosomal dominant conditions (Van Buchem disease type 2, Osteosclerosis/endosteal hyperostosis), and the autosomal dominant - recessive conditions (Sclerosteosis 1-2).
These disorders are a result of mutations in genes involved in disruption of the Wnt pathway, ultimately leading to an accumulation of β-catenin and activation of osteoblastic activity. Wnt signalling induces inhibition of the complex formed by four proteins (Axin, APC= adenomatous polyposis coli, GSK-3= glycogen synthase kinese 3, and b-catenin) in osteoblasts freeing B-catenin. B-catenin accumulates in the cytoplasm, then in the nucleus of the osteoblast, and ultimately acts as a cofactor to stimulate proliferation and differentiation of osteoblasts. LRP5 (lipoprotein receptor–related protein 5) is a co-receptor needed for activation of Wnt required for osteoblast activity. LRP5 is intermittently inhibited by its binding to another protein, Dickkopf-1 (DKK1). The binding of SOST (sclerostin) to LRP5 also inhibits Wnt signalling in osteoblasts. Defects of SOST or DKK1 binding lead to unchecked osteoblastic activity due to an inability to downregulate LRP5. All the mutations of LRP5 for these disorders are located in the region coding for the amino-terminal part of the LRP5 protein where LRP5 binds DKK1. The reduced affinity to this inhibitor of LRP5 results in a consititutive activation of the Wnt pathway and therefore increased bone formation. While inactivating mutations in the SOST gene cause sclerosteosis in van Buchem disease; a 52-kb deletion on chromsome 17q12 was identified that compromises a downstream enhancer of SOST.
Hyperostosis corticalis generalisata/Van Buchem disease is a rare skeletal dysplasia, that van Buchem et al. first described in 1955. This disease has an autosomal-recessive inheritance pattern and involves a genetic mutation in the SOST gene causing a defect in a downstream enhancer of sclerostin. Its distinguishing characteristics include progressive asymmetrical enlargement of the jaw that occurs during puberty, and overgrowth in the skull. Clinical complications including facial nerve palsy, optic atrophy, and impaired hearing occur in most patients.
Gene
SOST gene, 17q21.31 (OMIM gene/locus number #605740)
Phenotype
Progressive skeletal overgrowth (especially skull) and cortical thickening and generalized osteosclerosis, cranial hyperostosis, enlargement of the jaw with wide angle, dental malocclusion (uncommon), flat nasal bridge, frontal prominence, pain of long bone with applied pressure, no fragility fractures (long bones may become painful with applied pressure, but there are no fractures), hypertelorism, proptosis, multiple cranial nerve involvement with recurrent facial nerve palsy, headaches, deafness, optic atrophy from narrowing of cranial foramina.
Images
Fig. All patients showed the characteristic features of protruding chin, high forehead, and facial nerve paralysis (Van Hul W, Balemans W, Van Hul E, et al. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21. Am J Hum Genet. 1998 Feb;62(2):391-9).
Reproduced from Am J Hum Genet, Vol 62, Van Hul W, Balemans W, Van Hul E, et al. Van Buchem disease (hyperostosis corticalis generalisata) maps to chromosome 17q12-q21, Pages 391-9, Copyright 1998, with permission from Elsevier.
Main biochemical alterations
High bone ALP, high P1NP, decreased sclerostin in VBD, undetectable sclerostin in SOST1 and CDD
Osteopathia striata is characterized by linear striations at the ends of long bones and in the iliac bones that can remain unchanged for years. The striations are parallel to the long axis of the bone and are typically described in areas of rapid growth such as the femur.
(OMIM phenotype number #300373)
Osteopathia striata is characterized by linear striations at the ends of long bones and in the iliac bones that can remain unchanged for years. The striations are parallel to the long axis of the bone and are typically described in areas of rapid growth such as the femur. In particular, the striations in the iliac bones can present a fan-shaped appearance due to the growth pattern of these bones. Sclerosis of the skull, cranial nerve palsies are also common. Although an autosomal dominant inheritance was suspected, it appears to be an X-linked dominant disease caused by mutations in the WTX (Wilms tumor on the X chromosome) gene, an inhibitor of WNT signaling. Interestingly, whereas Wnt is somatically inactivated in 30% of Wilms tumor patients, no increased incidence of cancer is found in the osteopathia striata or in any other sclerosing bone disorder related to activation of the Wnt pathway. The pathogenesis of this disorder is still unknown. This disease presents in females with macrocephaly, cleft palate, mild learning disabilities, sclerosis of the long bones and skull, and longitudinal striations visible on radiographs of the long bones, pelvis, and scapulae. It is an X-linked dominant disease, but in hemizygous males it is usually associated with fetal or neonatal lethality. It is often accompanied by bilateral fibula aplasia.
Osteopathia striata with cranial sclerosis is a rare X-linked dominant sclerosing bone dysplasia characterized by longitudinal striations visible on radiographs of the metaphysis of the long bones, sclerosis of the craniofacial bones, macrocephaly, cleft palate and hearing loss. The phenotype is highly variable and vary from mild bone manifestations to multisystem organ involvement. Fewer than 100 cases have been reported up to now. OSCS follows an X-linked dominant pattern of inheritance, with frequent lethality in males.
Gene
AMER1 (WTX) gene, Xq11.2 (OMIM gene/locus number #300647)
Phenotype
Females: Long straight clavicole and striations visible on radiographs of the long bones, pelvis, and scapulae, sclerosis of the long bones and skull, sclerotic cranial base, sclerotic mastoids (31%), occipital bossing, thickened calvaria, bilateral fibula aplasia (always), increased trabecular thickness seen on iliac bone biopsy, joint contractures, trapezoidal shaped skull, thoracolumbar gibbus, scoliosis (23%), spina bifida occulta, absent or short fibulae, fifth finger clinodactyly, short stature and failure to thrive (in males), long and slender fingers, finger contractures, camptodactyly, duplicate phalanges spatulate, mild learning disabilities, macrocephaly, delayed closure of anterior fontanelle, large fontanelle, frontal bossing, widened sutures, bitemporal narrowing, micrognathia, hydrocephalus, headaches, speech delay (10%), transitional facial palsy (15%), hypotonia (in males), seizures (rare in males), mental retardation, mild-moderate (28%, usually in males), partial agenesis of corpus callosum (rare in males), hearing conductive loss, hypertelorism, cleft palate, ventricular septal defect, atrial septal defect, paranasal sinus hypoplasia, tracheomalacia, laryngeal web, broad and flat ribs, pectus excavatum, omphalocele (rare in males), multicystic kidney (rare in males), nemaline myopathy (in 1 reported male), intestinal malrotation (rare in males), anal stenosis (rare in males), anal atresia (rare in males), gastroesophageal reflux, fetal or neonatal lethality (male).
Osteosclerosis/endosteal hyperostosis, autosomal dominant, is a sclerosing bone disorder characterized by generalized skeletal densification, particularly of the cranial vault and tubular long bones without an increased risk of fracture (see also VBD).
(OMIM phenotype number #144750)
Osteosclerosis/endosteal hyperostosis, autosomal dominant, is a sclerosing bone disorder characterized by generalized skeletal densification, particularly of the cranial vault and tubular long bones without an increased risk of fracture (see also VBD). The syndrome has been described in less than 10 families and is due to a mutation in the Low-Density Lipoprotein Receptor-Related Protein-5 (LRP5) gene that leads to increased bone formation. The transmission is autosomal dominant. It can be distinguished from sclerostosis and VBD by a more benign clinical presentation, although the radiological characteristics can be overlapping.
Gene
LRP5 gene, 11q13.2 (OMIM gene/locus number #603506).
Phenotype
Increased bone density without sclerotic bands, mostly asymptomatic associated with osteosclerosis of the skull (cranial vault), enlarged and squared jaw (decreased gonial angle), no increased risk of fractures (fractures are unusual), cranial nerve compression, sensorineural hearing loss (otopharyngeal exostosis), cortical thickening of the long bones, torus palatinus (an osseous prominence of the palatal vault), normal height, flattened forehead (adolescence), malocclusion, tooth loss, elongated mandible, gonial angle decreased, mild rib sclerosis, mild clavicular sclerosis, mild vertebral body sclerosis, metacarpal and metatarsal diaphyseal endosteal sclerosis.
Main biochemical alterations
High ALP.
Buschke-Ollendorff syndrome is an autosomal dominant connective tissue disorder, characterized by multiple subcutaneous nevi or nodules (elastin-rich, elastoma, or collagen-rich, dermatofibrosis lenticularis disseminata, on histologic examination). Affected individuals also have osteopoikilosis. Osteopoikilosis is a disorder of endochondral ossification involving the secondary spongiosa.
(OMIM phenotype number #166700)
Buschke-Ollendorff syndrome is an autosomal dominant connective tissue disorder, characterized by multiple subcutaneous nevi or nodules (elastin-rich, elastoma, or collagen-rich, dermatofibrosis lenticularis disseminata, on histologic examination). Affected individuals also have osteopoikilosis. Osteopoikilosis is a disorder of endochondral ossification involving the secondary spongiosa. This disease literally means "spotted bones", which are osteosclerotic foci occurring in the epimetaphyseal region of long bones, such as wrist, foot, ankle, pelvis, and scapula. X rays show numerous small round or oval foci of osteosclerosis, as an incidental radiological finding. They may mimic metastatic lesions, but radionuclide accumulation is not increased on bone scans. Most reported cases of Buschke-Ollendorff syndrome and Osteopoikilosis are benign. The disease can be asymptomatic, or associated with skin manifestations. In some patients, melorheostosis can be present, characterized by asymmetric linear hyperostosis of the cortex of long bones, reminiscent of dripping candle wax, often associated with joint contractures, sclerodermatous skin lesion, muscle atrophy, hemangiomas, and limphedema. Inactivating mutations in the LEMD3 gene have been described. LEMD is a protein binding the inner nuclear membrane, which antagonizes TGF-β and bone morphogenic protein (BMP) signalling, resulting in focal deposits of compact lamellar bone in the spongiosa that have the appearance of an enostosis, or bone island. Multiple enostoses are laid down at the ends of short tubular bones, in the tarsal, carpal, and pelvic bones, and in the meta-epiphyseal regions of long bones.
Gene
LEMD3 gene, 12q14.3 (OMIM gene/locus number *607844)
Phenotype
Asymptomatic, disseminated connective tissue nevi with both elastic-type nevi (juvenile elastoma) and collagen type nevi (dermatofibrosis lenticularis disseminate), subcutaneous nontender firm nodules, subcutaneous connective tissue nevi, osteosclerotic foci in epimetaphyseal regions of long bones (wrist, foot, ankle, pelvis, and scapula), stiff joints, melorheostosis, typically affect diaphyses (less common).
Image
Fig. Anteroposterior radiograph of the left shoulder showing multiple osteopoikilosis lesions, best visible in the left humerus
Reproduced by permission from Macmillan Publishers Ltd: Nat Genet 2004 Nov;36:1213-8, copyright 2004.
Osteopetrosis is an inherited disorder with a variety of genetic causes, resulting in abnormally high bone mass for dysfunctional bone remodeling. This bone disease is divided into two main groups: autosomal recessive and autosomal dominant types.
(OMIM phenotype number #607634)
Osteopetrosis is an inherited disorder with a variety of genetic causes, resulting in abnormally high bone mass for dysfunctional bone remodeling. This bone disease is divided into two main groups: autosomal recessive and autosomal dominant types. The autosomal dominant form of osteopetrosis is less severe and often discovered in young to middle aged adults. There are two types of autosomal dominant forms. The osteopetrosis, autosomal dominant type 1 (OPTA1) is extremely rare and secondary to a defect of the LRP5 (low density lipoprotein receptor 5), causing an increased uniform sclerosis. The disease commonly has onset in late childhood or adolescence, and primarily involves the cranium. Clinical signs can include: chronic bone pain and disorders of the cranial nerves, such as trigeminal neuralgia, facial palsy, and hearing loss. Diagnosis is based on clinical and radiographic evaluation. Radiographically, the hallmark of osteopetrosis is increased density within the medullary portion of the bone with relative sparing of the cortices. In OPTA1, there is an uniform sclerosis of the long bones, skull, and spine.
The treatment is primarily supportive. In the future, the gene therapy may play a role in the future with an identifiable gene defect.
Gene
LRP5 gene, 11q13.2 (OMIM gene/locus number #603506).
Phenotype
Increased bone density, mostly asymptomatic (rarely bone pain).
Image
Fig. A patient affected by OPTA1. Chest radiograph obtained in an infant demonstrates overall increased density of the osseous
Reproduced from Scarsbrook Ihde LL, Forrester DM, Gottsegen CJ, et al., Sclerosing bone dysplasias: review and differentiation from other causes of osteosclerosis, Radiographics 2011;31:1865-82 with permission from The Radiographical Society of North America.
Other resources:
Hajdu-Cheney syndrome is a very rare connective tissue disorder, caused by heterozygous mutation in the NOTCH2 gene. It has autosomal dominant inheritance or in some cases may occur due to spontaneous de novo mutation.
(OMIM phenotype number #102500)
Hajdu-Cheney syndrome is a very rare connective tissue disorder, caused by heterozygous mutation in the NOTCH2 gene. It has autosomal dominant inheritance or in some cases may occur due to spontaneous de novo mutation. The disease has been associated with mutations in exon 34 of NOTCH2 upstream the PEST domain, leading to the creation of a truncated and stable NOTCH2 protein with enhanced NOTCH2 signaling activity. Notch receptors, single-pass transmembrane proteins determining cell fate, have an important role in skeletal development and homeostasis. Clinical manifestations include: transverse band of acro-osteolysis involving the phalanges of both hands and feet, severe osteoporosis short stature, deformities involving skull, mandible, spine and other bones, and wormian bones, neurological symptoms, and cardiovascular defects and polycystic kidneys. The comlications, that may occur are: kyphoscoliosis, basilar invagination, and bone fractures due to bone softening.
Treatment is symptomatic. Bone antiresorptive and anabolic agents have been tried to treat the osteoporosis, but their benefit has not been established.
Gene
NOTCH2 gene, 1p12p11 (OMIM gene/locus number #600275).
Phenotype
Short stature, coarse and dysmorphic facies, bowing of the long bones, vertebral anomalies Facial features include hypertelorism, bushy eyebrows, micrognathia, small mouth with dental anomalies, low-set ears, and short neck. Progressive focal bone destruction, including acroosteolysis and generalized osteoporosis. Additional and variable features include hearing loss, renal cysts, and cardiovascular anomalies.
Images
Fig. 43-year-old female patient with Hajdu-Cheney syndrome. Frontal radiograph of right hand (A) shows transverse band of osteolysis involving distal phalanx of second and third finger and middle phalanx of fifth finger. There is near complete osteolysis of distal phalanx of thumb. Marginal erosion is noted at distal interphalangeal joint of fourth finger. Radiograph of left hand (B) shows transverse osteolysis involving distal phalanx of thumb, second and third finger and middle phalanx of fifth finger. The fourth finger is spared. The soft tissues around the tips of terminal phalanges of both hands appear normal. No periarticular or any soft tissue calcification is seen in both hands.
Reproduced from J Radiol Case Rep. 2014 Sep 30;8(9):1-8 under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) License.
Melorheostosis, also called "Leri disease", is a type of mixed sclerosing bone dysplasia, with alterations in both endochondral and intramembranous ossification. The radiographic findings are characterized by hardened wax that has dripped down the side of a candle.
(OMIM phenotype number #155950)
Melorheostosis, also called "Leri disease", is a type of mixed sclerosing bone dysplasia, with alterations in both endochondral and intramembranous ossification. The radiographic findings are characterized by hardened wax that has dripped down the side of a candle. Dense, irregular and eccentric hyperostosis of both the cortex and the adjacent medullary canal of a single bone, or several adjacent bones have been described in this disorder. The bone lesions avidly accumulate radionuclide in a bone scan. The distribution of melorheostosis and its associated soft tissue lesions in sclerotomes, myotomes and dermatomes suggests a segmentary, embryogenetic defect. This may be an acquired defect related to spinal sensory nerves. Clinically, patients affected may have pain and stiffness of the involved bones, contractures when the osseous abnormality involves a joint, muscular atrophy and overlying skin changes. The skeletal lesions manifest in late childhood or early adulthood and progress during childhood and may or may not gradually extend in the adult years. Melorheostosis-like changes can be associated with osteopoikilosis. Less than 5 families have been reported in the literature to date. Osteopoikilosis is caused by a germline mutation in the LEMD3 gene (12q14), and a germline mutation in the LEMD3 gene may predispose individuals with osteopoikilosis to develop melorheostosis. However, the exact pathogenesis is currently unknown.
Differential diagnosis includes osteosarcoma in localised forms, myositis ossificans or calcified haematoma in cases associated with soft tissue calcifications. The therapeutic management is generally symptomatic, in case of severe deformity or contractures might be necessary surgery.
Gene
LEMD3 gene, 12q14.3 (OMIM gene/locus number #607844)
Phenotype
Joint contractures, contractures over affected bones, sclerodermatous skin lesions, skin atrophy over affected bones, sclerotic soft tissue over affected bones, muscle atrophy, hemangiomas, lymphedema, bone deformities with linear hyperostosis of the cortex of long bones reminiscent of dripping candle wax, flexion deformities over affected bones, flowing hyperostosis of bone cortex, osteosclerosis (lesions mainly affect diaphyses of long bones, hands, feet, and pelvis although epiphyses may also be affected).
Images
Fig. Radiograph shows: dense sclerosis involving the posterior cortex and adjacent medullary cavity of the proximal ulna with wavy margin. The elbow joint is not involved. The radius appeared normal.
Reproduced from J Chin Med Assoc, Vol 74, Abdullah S, Pang GM, Mohamed-Haflah NH, et al., Melorheostosis of the ulna., Pages 469-72, Copyright 2011, with permission from Elsevier.Osteoporosis-pseudoglioma syndrome, autosomal recessive, (OPPG) is a very rare disease caused by homozygous or compound heterozygous mutation in the gene encoding low density lipoprotein receptor-related protein-5 (LRP5), with loss of LRP5 function and decreased osteoblast activity.
(OMIM phenotype number #259770)
Osteoporosis-pseudoglioma syndrome, autosomal recessive, (OPPG) is a very rare disease caused by homozygous or compound heterozygous mutation in the gene encoding low density lipoprotein receptor-related protein-5 (LRP5), with loss of LRP5 function and decreased osteoblast activity. OPPG is characterized by congenital or infancy-onset blindness and extremely severe childhood-onset osteoporosis (lumbar spine Z-score often <-4) with spontaneous fractures. Radiographs disclose severe diffuse demineralization and often reveal fractures. The eye phenotype is secondary to defective vascularization and ranges from congenital phthisis bulbi to milder vitreoretinal changes. Ocular and skeletal abnormalities predominate, although other organs may be involved also. Other clinical manifestations may include: microphthalmos, abnormalities of the iris, lens or vitreous, cataracts, short stature, microcephaly, ligamental laxity, mental retardation and hypotonia. Usually mental development is normal but ∼25% have cognitive impairment. The phenotype is variable even among siblings. The prevalence is approximately 1/2.000.000.
Few data are available on the treatment of osteoporosis in OPPG, however some beneficial response to bisphosphonates have been described.
Gene
LRP5 gene, 11q13.1 (OMIM gene/locus number *603506)
Phenotype
Blindness, microphthalmia, vitreoretinal abnormalities, cataract, phthisis bulbi, absent anterior eye chamber, iris atrophy, pseudoglyoma, muscle hypotonia, obesity, mental retardation (in some cases), ligament laxity, severe osteoporosis, multiple fractures, short stature, kyphoscoliosis, hyperextensible joints, and wide metaphyses.
Images
Fig. (a) Persistence of the fibrovascular system (pseudoglioma) visible upon funduscopy. (b) Standard radiograph of the lumbar spine in a 37-year-old man with OPPG. Note the extremely severe demineralization and multiple vertebral fractures (Levasseur R, Lacombe D, de Vernejoul MC. LRP5 mutations in osteoporosis-pseudoglioma syndrome and high-bone-mass disorders. Joint Bone Spine. 2005 May;72(3):207-14.).
Reproduced from Br J Ophthalmol, Wilson G, Moore A, Allgrove J, volume 85, page 1139, copyright notice 2001 with permission from BMJ Publishing Group Ltd.
Other resources:
Camurati-Engelmann Disease Type II, called also "Progressive diaphyseal dysplasia with striations of the bones", is a rare sclerosing bone dysplasia. Until now, few clinical cases have been reported.
(OMIM phenotype number %606631)
Camurati-Engelmann Disease Type II, called also "Progressive diaphyseal dysplasia with striations of the bones", is a rare sclerosing bone dysplasia. Until now, few clinical cases have been reported. This disorder is characterized by bone striations, manifest radiologically, metadiaphyseal expansion with cortical thickness of the tubular bones, and coarse, thick bony trabeculae of the tubular bones, spine, flat bones and minimal sclerosis in the petromastoid region. Serum alkaline phosphatase (ALP) levels are high. Patient affected by this disorder are usually asymptomatic or have mild limb pains. Autosomal dominant transmission has been proposed, but affected males outnumber affected females (see also CAEND).
Gene
TGF-β1 gene, 19q13.2 (OMIM gene/locus number *190180)
Phenotype
Marfanoid habitus, waddling gait, muscular weakness, intense leg pain, flexion contracture of the hip and knee joints, delayed sexual development, cortical thickening of the diaphyses. Metaphyseal expansion of the long bones, coarse and thick trabeculae of the long and short tubular bones, striations in the spinal, pelvic, and long bones, and cranial sclerosis restricted to the petromastoid regions.
Main biochemical alterations
High ALP, high ESD.
Other resources:
Osteogenesis imperfecta with calcification in interosseous membranes and/or hypertrophic callus (OI type V) is a moderate form of Osteogenesis Imperfecta (see also OI type I). It is a genetic disorder characterized by increased bone fragility, low bone mass and susceptibility to bone fractures with variable severity.
(OMIM phenotype number #610967)
Osteogenesis imperfecta with calcification in interosseous membranes and/or hypertrophic callus (OI type V) is a moderate form of Osteogenesis Imperfecta (see also OI type I). It is a genetic disorder characterized by increased bone fragility, low bone mass and susceptibility to bone fractures with variable severity. Clinical findings of OI type V include mild to moderate short stature, dislocation of the radial head, mineralized interosseous membranes, white sclera and no dentinogenesis imperfecta. Patients affected by OI type V can have hypertrophic callus, dense metaphyseal bands and/or ossification of the interosseus membranes of the forearm, causing severely limited pronation and supination. Recently, it was found that cases of OI type V are caused by the same recurring defect in the IFITM5 gene that encodes the BRIL (Bone-restricted IFITM-like) protein, a known osteoblast marker (highly expressed in mineralizing osteoblasts).
Gene
IFITM5 gene, 11p15.5 (OMIM gene/locus number #614757).
Phenotype
Moderate-severe OI form, similar to type IV, but without dentinogenesis imperfecta and blue sclerae, calcification of intraosseus membranes in the forearm and hyperplastic callus formation, metaphyseal bands adjacent to growth plate (distal femora, proximal tibia, distal radii), and histological mesh-like or irregular bone pattern.
Main biochemical alterations:
High ALP, and high NTX.
Images