ALTERED OSTEOCLAST, OSTEOBLAST OR OSTEOCYTE ACTIVITY
High Bone Resorption
Cystic angiomatosis is a nonaggressive form of skeletal angiomatosis with multifocal hemangiomatous and/or lymphangiomatous lesions of the skeleton, predominantly affecting the trunk bones, with possible visceral organ involvement. The exact pathogenetic mechanism of the disease is not still clear.
(OMIM #123880)
Cystic angiomatosis is a nonaggressive form of skeletal angiomatosis with multifocal hemangiomatous and/or lymphangiomatous lesions of the skeleton, predominantly affecting the trunk bones, with possible visceral organ involvement. The exact pathogenetic mechanism of the disease is not still clear. The disorder can affect any part of the skeleton, mostly the pelvis, long bones, and shoulder girdles being. The sites of extraskeletal involvement include: soft tissues, lungs, liver, and spleen. The most severe form of angiomatosis is represented by Gorham–Stout disease (see also GSD). The disease is usually manifested by the age of puberty to the third decade or over the age of 50. The process can be monostotic or polyostotic, and isolated skeletal involvement is very rare. The diagnosis is difficult to establish and partly one of exclusion. Laboratory findings are unremarkable, except increases in alkaline phosphatase (ALP) activity. Histologically GSD may be identical to CA, but GSD causes significantly more destruction with osteolysis and tends to involve one bone only. Clinical symptoms, are poor and include: loss of strength, pain, skin pallor, swelling of the affected area, functional difficulties such as difficulty in walking in the case of involvement of the lower limbs, shortening and bowing of an extremity, and scoliosis. Moreover, the disease can be complicated by vertebral localization or chylothorax. X-rays show lytic lesions that appear to contain residual lamellar bone and foci of reactive woven bone. The bone biopsy is always accompanied by intraosseous non-malignant proliferation of thin vascular structures which results in increased bone resorption. These lesions are, usually, progressively replaced by hypervascular extensive fibrosis. This process can continue for years and may stop spontaneously. Prognosis and complications vary widely depending on the site and extent of bone destruction and the visceral involvement. The typical course of CA is relatively benign, and not incompatible with a long life span; but sometimes there can be a dangerous involvement of vital structures.
The treatments of CA include radiation therapy, surgical procedures, and/or medical therapy (aminobisphosphonates, interferon-a, and calcitonin). However, there is no universally recognized successful treatment.
Phenotype
Multifocal hemangiomatous and/or lymphangiomatous lesions of the skeleton with possible visceral organ involvement.
Main biochemical alterations
High OPG, high OPN, high IL-6, ALP slightly high.
Images
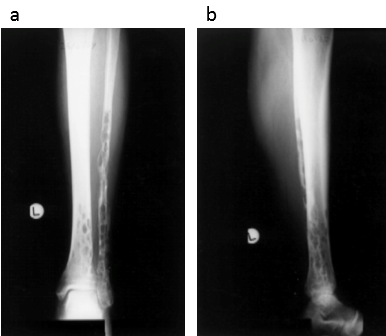
Fig. (a) Anteroposterior and (b) lateral radiographs of the tibia and fibula with the characteristic ‘swiss cheese’ appearance of the affected bones
Reproduced from Clayer M, Skeletal angiomatosis in association with gastro-intestinal angiodysplasia and paraproteinemia: a case report. J Orthop Surg (Hong Kong) 2002;10:85-8.
Cystic angiomatosis of bone/Gorham-Stout disease (GSD), (also known as massive osteolysis, vanishing bone disease, and phantom bone disease) is a rare disease characterized by the proliferation of endothelial-lined vessels in bone, resulting in the destruction of osseous matrix and absorption of bone and possible visceral involvement.
(OMIM phenotype number #123880)
Cystic angiomatosis of bone/Gorham-Stout disease (GSD), (also known as massive osteolysis, vanishing bone disease, and phantom bone disease) is a rare disease characterized by the proliferation of endothelial-lined vessels in bone, resulting in the destruction of osseous matrix and absorption of bone and possible visceral involvement. GSD can present at any age but is most commonly diagnosed in children and young adults (average age of diagnosis is 25 years). This disorder does not display a clear race, geographic distribution and sex predilection (1.6:1 male-to-female ratio) or inheritance pattern. More than 300 cases of GSD have been described in the literature. The etiology is unknown, although several theories exist. One or multiple bones can be affected, including the skull, the upper and lower extremities, the spine and pelvis. The clinical manifestations are, most frequently: pain, functional impairment, and swelling of the affected region. Nevertheless, some asymptomatic cases have been described. The disease has a benign character, but its prognosis is unpredictable and there can be physical deformities, disabilities, and several possible serious complications (pleural effusion, chylothorax, hemangiomatosous cutaneous lesions, bone infection and subsequent septic shock, spinal cord involvement and paraplegia due to vertebral lesion and cerebrospinal fluid leakage and meningitis). The differential diagnosis of the GSD includes: hereditary multicentric osteolysis, osteolysis with nephropathy, osteomyelitis, rheumatoid arthritis, osteolysis due to intraosseous malignacies, hyperparathyroidism, eosinophilic granuloma and osteolysis due to diseases of central nervous system, like syringomyelia and tabes dorsalis.
The diagnosis of the syndrome is challenging. Laboratory findings are not indicative except alkaline phosphatase (ALP), which may be slightly high. Moreover, some studies have shown that VEGF-A and IL-6 concentrations can be high in the circulation of patients affected by GSD and that the level of these factors can decrease after some treatments. However, VEGF-A and IL-6 are not elevated in all GSD patients. Plain X-rays, initially, show radiolucent foci in the intramedullary or subcortical regions and, later, slowly progressive atrophy, dissolution, fracture, fragmentation and disappearance of a part of a bone. On the other side, bone scan and magnetic resonance imaging give variable results. The disease is confirmed by the histopathological analysis of the lesions, showing nonmalignant hyperproliferation of small vessels. Heffez et al suggested the following 8 diagnostic criteria of Gorham-Stout syndrome: (1) positive biopsy findings in terms of angiomatous tissue presence; (2) absence of cellular atypia; (3) minimal or no osteoclastic response and absence of dystrophic calcifications; (4) evidence of local bone progressive resorption; (5) non-expansive, non-ulcerative lesion; (6) absence of visceral involvement; (7) osteolytic radiographic pattern; and (8) negative hereditary, metabolic, neoplastic, immunologic and infectious etiology.
The therapeutic options for individuals with GSD are limited and include: medicine therapy (biphoshonates, interferon alfa-2b..), radiation and surgery (resection of the lesion and reconstruction by use of bone grafts and/or prostheses, pleurectomy, pleurodesis, thoracentesis...). Biphoshonates have been successfully used showing an antiosteolytic activity. Some individuals have been treated with interferon alfa-2b, which inhibits the formation of lymphatic vessels (anti-angiogenic). Besides, other pharmacologic agents, like vitamin D, calcium, adrenal extracts and androgens have been suggested. Further clinical trials are needed to test the efficacy of therapies against GSD.
Gene
GSD is a sporadic disease potentially caused by specific genetic risk factors or by mosaicism for a somatic mutation.
Phenotype
Inherited osteolysis disorder characterized by destruction and resorption of affected bones with subsequent skeletal deformities and functional impairment. Early-onset progressive osteolysis of one or more bones always associated with an angiomatosis of blood vessels and sometimes of lymphatics, history of fragility fractures, and vascular malformations in the affected bones or surrounding soft tissues, multiple dilated vascular spaces replacing normal bone marrow elements, disseminated multifocal vascular lesions of the skeleton with possible visceral involvement, bony deformities, muscular weakness and localized pain.
Main biochemical alterations
High IL-6 (not always), high serum fibrinogen, high serum D-dimer, high ESR, high CD105/endoglin, ALP slightly high.
Images
Fig. Radiographs show (a) the right hip of a 77-year-old woman ten weeks after a fall, with complete resorption of the proximal femur, (b) the right shoulder of a 83-year-old woman with massive osteolysis of the proximal humerus, and (c) the right shoulder of a 56-year-old woman with disappearance of the head of the right humerus leaving only bone fragments.
Reproduced with permission and copyright © of the British Editorial Society of Bone and Joint Surgery [Möller G, Priemel M, Amling M, et al. The Gorham-Stout syndrome (Gorham's massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999 May;81(3):501-6].
Craniodiaphyseal dysplasia is a severe form of bone dysplasia, characterized by massive generalised hyperostosis and sclerosis, primarly involving the facial bones and the skull, leading to severe deformity.
(OMIM phenotype number #122860)
Craniodiaphyseal dysplasia is a severe form of bone dysplasia, characterized by massive generalised hyperostosis and sclerosis, primarly involving the facial bones and the skull, leading to severe deformity. Patients with CDD have facial abnormalities in early infancy. During childhood, progressive bony encroachment upon cranial foramina leads to severe neurological impairment. The bone deposition results in progressive stenosis of craniofacial foramina and facial distortion (leonine facies). In particular, infants affected by CDD may have respiratory difficulty owing to nasal obstruction before the characteristic facial appearance has developed. It has been observed heterozygous mutations located in the secretion signal of the SOST gene in two CDD patients and demonstrated that these SOST mutations prevent sclerostin secretion resulting in increased bone formation.
Gene
SOST gene, 17q21.31 (OMIM gene/locus number #605740) (see also VBD)
Phenotype
Leonine facies, increased bone mineral density, hyperostosis, osteosclerosis, obliteration of the sinuses, middle ear cavities, internal acoustic canals, and optic nerve canals, progressive hearing loss, progressive visual loss (cranial nerve compression, especially of II and VIII), cortical sclerosis of facial bones, macrocephaly, prominent mandible, facial diplegia, strabismus, exophthalmus, hypertelorism, papilledema, saddle nose, broad flat nasal bridge, choanal stenosis, increased intracranial pressure, headaches, seizures, mental retardation (however, in the majority of cases developmental progress was normal until hindered by progressive deterioration in vision and hearing), diaphyseal sclerosis, thickened and sclerotic ribs, undertubulation of the long bones of the legs, short stature (growth may be markedly retarded and delayed sexual maturation has been reported), difficulty breathing through the nostrils, and respiratory obstruction.
Images
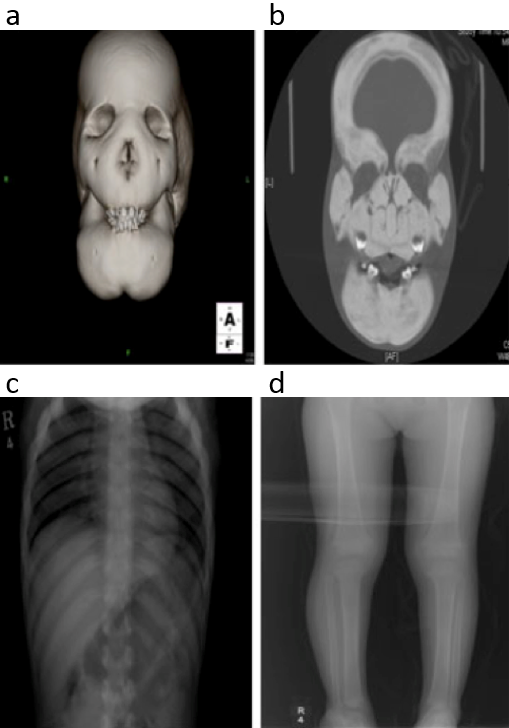
Fig. a,b,c,d. Radiological findings of a case with CDD at the age of 3 years. There are marked thickening and sclerosis of the skull and whole facial bones. The ribs are also thickened and sclerotic. Mild undertubulation of the long bones of leg is noted.
Reproduced from H Genet, Identification of signal peptide domain SOST mutations in autosomal dominant craniodiaphyseal dysplasia, 2011;129:497-502, Kim SJ, Bieganski T, Sohn YB, Kozlowski K, et al., with permission of Springer.
The autosomal recessive form of Craniometaphyseal dysplasia (see CMD) is less common and more severe than the dominant. Diaphyseal sclerosis is more apparent in the recessive form and can be found in adults.
(OMIM phenotype number #218400)
The autosomal recessive form of Craniometaphyseal dysplasia (see CMD) is less common and more severe than the dominant. Diaphyseal sclerosis is more apparent in the recessive form and can be found in adults. Cranial nerve deficits have been reported in infancy and earlychildhood in a few patients, but the evolution of the findings in recessive CMD is not well documented.
Gene
GJA1 gene, 6q22.31 (OMIM gene/locus number #121014), encoding for the gap junction alpha 1 protein and is also known as Connexin 43, CX43.
Phenotype
Hyperostosis and sclerosis of the craniofacial bones and abnormal modeling of the metaphyses, sclerosis of the skull, asymmetry of the mandible, cranial nerve compression with hearing loss, facial palsy, macrocephaly, coarse facial features, dystopia canthorum, hypertelorism, optic atrophy, broad nasal bridge, bony paranasal bossing, widened alveolar ridges, delayed eruption of permanent teeth, nasal obstruction leading to mouth breathing, mild anterior rib widening, obliteration of paranasal sinuses and mastoid, gene valgum, dense diaphyses, metaphyseal flaring, club-shaped distal femora, humeri, radii, ulnae bowing, metacarpal sclerosis, phalangeal sclerosis.
Other resources
Familial hydiopatic hyperphosphatasia, also called juvenile Paget’s disease of bone, is a rare autosomal recessive juvenile-onset form of Paget disease, characterized by markedly accelerated bone turnover caused by osteoprotegerin deficiency (for inactivating homozygous or compound heterozygous mutation in the TNFRSF11B gene).
(OMIM phenotype number #239000)
Familial hydiopatic hyperphosphatasia, also called juvenile Paget’s disease of bone, is a rare autosomal recessive juvenile-onset form of Paget disease, characterized by markedly accelerated bone turnover caused by osteoprotegerin deficiency (for inactivating homozygous or compound heterozygous mutation in the TNFRSF11B gene). This disorder is characterized by: an increase in bone turnover (elevated levels of serum alkaline phosphatase (ALP) and other markers of bone turnover), skeletal deformity, bone expansion, bone pain and an increased risk of pathological fractures. Clinical manifestations present from early infancy and include: skeletal deformity and failure to thrive followed by skull enlargement, difficulty in walking, progressive sensorineural deafness (due to cochlear involvement), kyphosis and acetabular protrusion. Disease severity generally increases during adolescence, but a milder form has been described in some patients.
Currently, there are not guidelines of therapeutic management for this rare disease. Nervetheless, there are some studies on the use of antiresorptive drugs such as calcitonin, bisphosphonates and denosumab, with improvement of clinical, biochemical and radiographic. Recombinant OPG has also been used successfully in treatment, but currently this is not available for routine clinical use.
Gene
TNFRSF11B gene, 8q24.12 (OMIM gene/locus number *602643), which encodes osteoprotegerin (OPG), a member of the TNF-receptor superfamily. OPG is a soluble decoy receptor for RANKL which inhibits osteoclast differentiation and bone resorption.
Phenotype
Muscular weakness, deafness in infancy, osteoporosis, expanded long bones, bowed long bones, fragile bones, increased bone formation and destruction, progressive skeletal deformity, short stature, mild involvement of cranial bones, islands of increased skull bone density, premature teeth loss, retinal degeneration in some individuals, and angioid streaks.
Main biochemical alterations
High Pi, normal Ca, markedly high ALP, high acid phosphatase, high uric acid.
Images
Fig. Radiographs from patient 1 at age 9 months, showing characteristic features of juvenile Paget’s disease of bone. (a) and (b) expanded and bowed femoral diaphysis, thin cortices, periosteal new bone formation, and disorganized trabecular architecture with (c) similar appearances in the humerus.
Reproduced from Bone, Vol 68, Naot D, Choi A, Musson DS et al. Novel homozygous mutations in the osteoprotegerin gene TNFRSF11B in two unrelated patients with juvenile Paget's disease, Pages 6-10 Copyright 2014, with permission from Elsevier.
Other resources
Familial expansile osteolysis (FEO) is an extremely rare, autosomal dominant, bone dysplasia. Clinical manifestations include: early-onset deafness, tooth loss and progressive lytic expansion within several limb bones causing bone pain, deformity, and fracture, associated with high serum alkaline phosphatase (ALP) and urinary hydroxyproline (Ur OHP) concentrations.
(OMIM phenotype number #174810)
Familial expansile osteolysis (FEO) is an extremely rare, autosomal dominant, bone dysplasia. Clinical manifestations include: early-onset deafness, tooth loss and progressive lytic expansion within several limb bones causing bone pain, deformity, and fracture, associated with high serum alkaline phosphatase (ALP) and urinary hydroxyproline (Ur OHP) concentrations. Initially, the focal skeletal defects have an active osteolytic phase, similar to Paget disease of bone, but evolve to expanded, shell-like bones that are fat filled. In osteoclasts from affected bone by FEO have been identified nuclear inclusion bodies identical to those found in PDB. Bone lesions have been observed mostly in the lower leg, forearm, hand and foot bones but rarely in the axial skeleton.
The management for FEO is unclear, but inhibitors of osteoclastic bone resorption (bisphosphonates and calcitonin), are effective at reducing biochemical markers of disease activity and improving bone pain. It has not yet been described whether bisphosphonate therapy alters natural history of the disease or prevents complications.
Gene
TNFRSF11A gene, 18q21.33 (OMIM gene/locus number #603499), encoding receptor activator of NFkB (RANK), which binds RANK ligand. RANK and RANKL (RANK ligand) have a fundamental physiological role in the regulation of osteoclast differentiation.
Phenotype
Deafness and loss of dentition, focal skeletal changes, with predominantly peripheral distribution, progressive osteoclastic resorption accompanied by medullary expansion led to severe, painful, disabling deformity and a tendency to pathologic fracture.
Main biochemical alterations
High ALP, high Ur OHP, possible high Ca.