There are a number of other methods for assessing osteoporosis and/or fracture risks that have been used extensively in clinical trials and epidemiological studies. These include radiological assessments and Bone Turnover Markers (BTM), as well as tools to assess fracture risk.
DXA-based tools used to assess bone structure
Two other DXA-based tools can measure bone macro- and micro-structure: vertebral fracture assessment (VFA), which is discussed in assessing fragility fractures, and trabecular bone score (TBS) below.
Trabecular Bone Score (TBS)
TBS is a grey-level textural measurement usually acquired from conventional lumbar spine DXA BMD images. It captures information on trabecular microarchitecture related to 3D bone characteristics (e.g., trabecular number, connectivity density). It provides a validated index of bone microarchitecture, which correlates with the mechanical properties of bone [1]Shevroja E et al., Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos Int. 2023 34(9): p1501-1529.
. A version of the software v4.0 corrects for regional tissue thickness [2]Gatineau G et al., Advancing trabecular bone score (TBS): clinical performance of TBS version 4.0 with direct correlation for soft tissue thickness-the osteolaus study. Osteoporos. Int. 2025
. TBS can be used alongside BMD to enhance the assessment of fracture risk and inform treatment decisions and monitoring in osteoporosis.
The clinical use of TBS has been recognised in the following cases, among others:
1. Enhanced fracture risk prediction:
- TBS predicts hip and major osteoporotic fractures independently of BMD and clinical risk factors (CRFs) in postmenopausal and male osteoporosis[3]Shevroja E et al., The fracture predictive ability of lumbar spine BMD and TBS as calculated based on different combinations of the lumbar spine vertebrae. Arch Osteoporos. 2022.17(1): p83.
. - TBS has been integrated into FRAX and is a predictor of fracture risk independent of FRAX[4]McCloskey EV et al., A meta-analysis of trabecular bone score in fracture risk prediction and its relationship to FRAX. J Bone Miner Res 2016 31(5): p940–948.
. - TBS-adjusted BMD T-score is an alternative solution.
- TBS enhances fracture risk prediction in secondary osteoporosis, including type 2 diabetes, glucocorticoids, CKD, rheumatological diseases[1]Shevroja E et al., Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos Int. 2023 34(9): p1501-1529.
.
2. Treatment decision-making:
- TBS can guide the initiation and choice of anti-osteoporosis treatment, especially for individuals near intervention thresholds, when taken with BMD and CRFs [1]Shevroja E et al., Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos Int. 2023 34(9): p1501-1529.
. - It helps in stratifying patients for anabolic-first approaches in very high-risk cases[1]Shevroja E et al., Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos Int. 2023 34(9): p1501-1529.
.
3. Monitoring treatment efficacy:
- TBS is useful for monitoring long-term response to denosumab and anabolic agents[5]Hans D et al., Long-term effect of denosumab on bone microarchitecture as assessed by tissue thickness–adjusted trabecular bone score in postmenopausal women with osteoporosis: results from FREEDOM and its open-label extension. Osteoporos Int. 2023 34(6): p1075–1084.
[6]Bilezikian JP et al., Abaloparatide-SC improves trabecular microarchitecture as assessed by trabecular bone score (TBS): a 24-week randomized clinical trial. Osteoporos Int. 2018 29: p323–328.
.
Clinical use of artificial intelligence in fracture risk assessment and diagnosis of osteoporosis
Artificial intelligence (AI)-based programmes are producing good diagnostic results in fracture detection from X-ray or CT images. AI can accurately diagnose and predict the risk of osteoporotic vertebral fracture and vertebral compression fracture[7]Namireddy SR et al., Artificial intelligence in risk prediction and diagnosis of vertebral fractures. Sci Rep. 2024 14(1): p30560.
. At this stage, future efforts should focus on standardising AI models and validating them across diverse datasets to ensure their clinical utility. AI holds great promise as a diagnostic adjunct in clinical practice.
Other tools to measure Bone Mineral Density (BMD)
The most commonly used and recommended tool to measure BMD is DXA. There are other types of test options, however, these are not all recognised by healthcare systems for the diagnosis of osteoporosis:
- QCT (Quantitative Computed Tomography) measures the spine or hip
- pQCT (peripheral QCT) measures the forearm and tibia
- QUS (Quantitative Ultrasound) uses sound waves to measure the heel or finger
- REMS (Radiofrequency Echographic Multi Spectrometry) technology
To date, Quantitative Computed Tomography (QCT) is mainly used as a research tool due to the significantly higher X-ray dose compared to DXA. With recent developments, peripheral QCT permits lower exposures measuring bone indices in the limbs (tibia, radius/ulna) [8]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
. CT assessment generates true volumetric measures of bone, and thus complement DXA, which yields a 2D representation of the region of interest [9]Genant HK, et al., Noninvasive assessment of bone mineral and structure: state of the art. J Bone Miner Res, 1996. 11(6): p. 707-30.
. In contrast with DXA and to allow results to be in calcium hydroxyapatite equivalent BMD, the reference phantom (known composition) is scanned at the same time as the patient [8]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
, although newer algorithms do not require this [10]Therkildsen J, et al., Vertebral Bone Mineral Density Measured by Quantitative Computed Tomography With and Without a Calibration Phantom: A Comparison Between 2 Different Software Solutions. J Clin Densitom, 2018. 21(3): p. 367-374.
. Another research tool is high-resolution peripheral QCT (HR-pQCT) that can provide detailed microstructural imaging, yielding an image equivalent to a “virtual bone biopsy” [11]Boutroy S, et al., In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab, 2005. 90(12): p. 6508-15.
.
Also frequently used to assess bone mass is quantitative ultrasound (QUS). However, clinical use of QUS is restricted to the heel, where it can predict fragility fracture risk, independent of axial BMD, in postmenopausal women and older women. Bone structure may also be determined, yet its clinical utility is minimal [8]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
Radiofrequency Echographic Multi-Spectrometry (REMS) is a relatively recent technology that analyses bone quantity and quality through a non-ionising approach based on the analysis of ultrasound signal backscattering [12]Diez-Perez A, et al., Radiofrequency echographic multi spectrometry for the in vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organised by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), Aging Clin. Exp. Res 2019; 31: p. 1375–1389.
[13]Casciaro S, et al., An advanced quantitative echosound methodology for femoral neck densitometry, Ultrasound Med. Biol. 2016; 42(6):p. 1337–1356.
[14]Conversano F, et al., A novel ultrasound methodology for estimating spine mineral density, Ultrasound Med. Biol. 2015; 41 (1)p. 281–300.
. BMD is calculated through comparisons of the patient’s specific echographic spectrum of the target bone against a proprietary database of reference ultrasound spectral models, and the corresponding T-score and Z-score values are derived using a normative reference database, i.e., the National Health and Nutrition Examination Survey (NHANES) [12]Diez-Perez A, et al., Radiofrequency echographic multi spectrometry for the in vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organised by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), Aging Clin. Exp. Res 2019; 31: p. 1375–1389.
.
Tools to assess fracture risk
The measurement of BMD is a diagnostic tool for osteoporosis, but also a fracture risk assessment tool. However, in terms of risk assessment, BMD should be one of many factors considered as there are many other skeletal and non-skeletal elements that determine bone strength and therefore fracture risk. This can be illustrated by the fact that most fragility fractures happen in individuals with osteopenic BMD values, as opposed to being below the osteoporosis threshold [15]World Health Organization Study Group - Technical Report Series 843: Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. 1994 [Accessed 22.02.2019]
. As such, research has tried to identify other factors that can be linked to an increased fracture risk. These risk factors are all linked to a greater or lesser degree to BMD and can thus complete information from a BMD test or can be used to assess fracture risk without BMD results [16]Kanis J. et al, Assessment of osteoporosis at the primary health-care level. WHO Scientific Group Technical Report. 2007 [Accessed 22.02.2019]
. This led to the development of fracture prediction tools (see table below), which are increasingly present in clinical practice as despite their limitations, they all achieve better results compared to the use of a unique risk factor.
Bone Turnover Markers (BTM)
Bone Turnover Markers (BTM) can be measured in urine and/or blood samples, however, serum samples are the gold standard for quantification [17]Bhattoa HP, Laboratory aspects and clinical utility of bone turnover markers. EJIFCC, 2018. 29(2): p. 117-128.
. These markers represent powerful research tools for epidemiologists studying fracture risk across populations and have been extensively used in clinical research to monitor the efficacy and mechanisms of action of new drugs. Combining BMD with BTM could improve fracture prediction in postmenopausal women [18]Delmas PD, et al., The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int, 2000. 11 Suppl 6: p. S2-17.
[19]Johnell O, et al., Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int, 2002. 13(7): p. 523-6.
.
However, the diagnosis value of BTMs at baseline in osteoporosis is very low. As they are not able to confirm the presence or absence of osteoporosis, BTMs cannot be considered as a substitute for BMD testing. Their use for identifying patients at risk of rapid bone loss is also limited.
In contrast, the use of BTMs to guide osteoporosis therapy has a clearer potential utility. This is particularly the case for the serum amino-terminal propeptide of type I collagen (PINP), marker of bone formation, and the serum carboxy (C)-terminal telopeptide (CTX), a marker of bone resorption [20]Morris HA, et al., Clinical usefulness of bone turnover marker concentrations in osteoporosis. Clin Chim Acta, 2017. 467: p. 34-41.
[21]Vasikaran S, et al., International Osteoporosis Foundation and International Federation of Clinical Chemistry and Laboratory Medicine position on bone marker standards in osteoporosis. Clin Chem Lab Med, 2011. 49(8): p. 1271-4.
. The pattern of change in BTMs in response to treatment is well described and significant changes in BTM can be seen during antiresorptive therapy after a few weeks of treatment, whereas individual monitoring with DXA usually requires 1-2 years to identify significant changes. As adherence is an important issue of long-term therapy in chronic disease, it has been suggested that BTMs could be used in clinical practice to assess the patient's adherence to treatment and also provide feedback on the effectiveness of the medication [22]Diez-Perez A, et al., International Osteoporosis Foundation and European Calcified Tissue Society Working Group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int, 2017. 28(3): p. 767-774.
.
Fracture Risk Assessment Tool (FRAX®)
The FRAX® tool was developed by the then WHO Collaborating Centre for Metabolic Bone Diseases (1991-2010) at the University of Sheffield. It was launched in 2008 following approximately 10 years of meta-analyses of a variety of risk factors for osteoporotic fracture. Although not the only fracture prediction tool available, FRAX® is the only risk calculator which has been calibrated to rates of fracture and mortality per individual country and has been shown to identify a risk amenable to available treatments.
Recently, research has demonstrated that actively screening older women for fracture risk using FRAX in the primary care setting leads to a reduction in the risk of hip fracture [23]Chotiyarnwong P, McCloskey, EV, Harvey NC, et al., Is it time to consider population screening for fracture risk in postmenopausal women? A position paper from the International Osteoporosis Foundation Epidemiology/Quality of Life Working Group. Arch Osteoporos, 2022. 17(87)
.
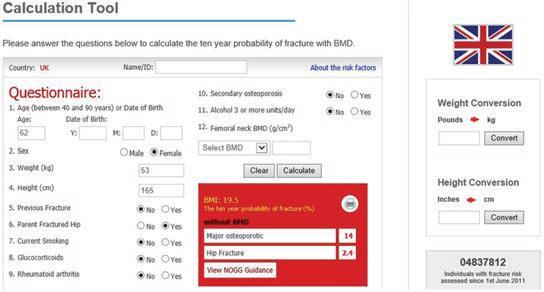
FRAX® is a computer-based algorithm that calculates the 10-year probability of a major osteoporotic fracture (hip, clinical spine, humerus or wrist fracture) and the 10-year probability of hip fracture alone. The easy-to-use tool helps physicians calculate an individual patient’s fracture risk based on age, body mass index and well-validated risk factors. Femoral neck bone mineral density (BMD) and trabecular bone score (TBS) can be optionally input to enhance fracture risk prediction.
Fracture probability differs greatly in different regions of the world. FRAX® models are available for those countries where the epidemiology of fracture and death is known (January 2019: 68 models for 64 countries, in 31 language options). In the absence of a FRAX® model for a specific country, a surrogate country can be chosen, based on the likelihood that it is representative of the index country.
FRAX® has been independently evaluated to be effective and robust, and it has been approved by the National Institute for Health and Care Excellence (UK) and the Food and Drug Administration (USA). As well, a recent study has also shown the potential of FRAX® as an effective screening tool, which showed a 28% reduction in hip fractures [24]Shepstone, L., et al., Screening in the community to reduce fractures in older women (SCOOP): a randomised controlled trial. Lancet, 2018. 391(10122): p. 741-747.
.
Figure taken from Ferrari & Roux, 2019 [8]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
In addition to the freely accessible web-based FRAX® calculator, FRAX® is also available in other formats, including in densitometers.
In clinical practice, FRAX® is intended to help clinicians make more informed diagnosis and treatment decisions about who and when to treat. Some guidelines recommend the use of FRAX® prior to BMD measurement (e.g. UK, Europe) and some recommend BMD measurement first (e.g. the USA), dependent on whether DXA equipment is available.
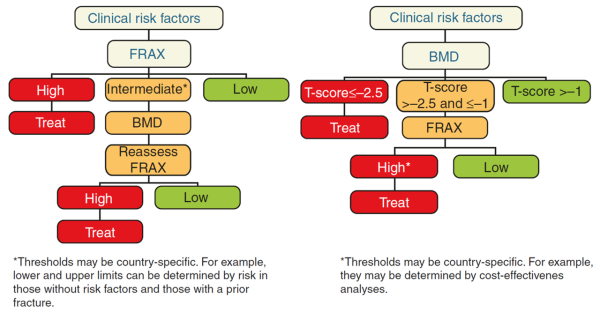
The risk of fracture determined by FRAX® can be used by the clinician in deciding next steps, in accordance with national or academic assessment guidelines and treatment threshold recommendations (if provided). In most guidelines, treatment for osteoporosis is recommended in individuals with prior fragility fractures, especially fractures at the spine and hip. However, for those without prior fractures, the intervention thresholds differ across guidelines. Following the assessment of fracture risk using FRAX® in the absence of BMD, the patient may be classified to be at low, intermediate or high risk.
Figure taken from Ferrari & Roux, 2019 [8]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
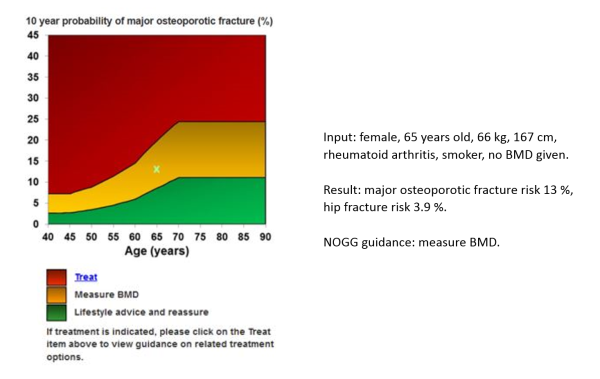
Below is the UK example of how a FRAX® result is used to enhance decision-making:
Figure taken from FRAX calculation 31.01.2019; National Osteoporosis Guideline Group (NOGG) updated guidance: https://www.sheffield.ac.uk/NOGG/index.html
- Low risk – reassure, give lifestyle advice, and reassess in 5 years or less depending on the clinical context.
- Intermediate risk – measure BMD and recalculate the fracture risk to determine whether an individual's risk lies above or below the intervention threshold.
- High risk – can be considered for treatment without the need for BMD, although BMD measurement may sometimes be appropriate, particularly in younger postmenopausal women.
It is important to note that FRAX® thresholds are for guidance only, and the decision as to whether to reassess or treat lies with the clinician. Good clinical judgement should take into account FRAX’s limitations [25]Kanis, J.A., et al., A systematic review of intervention thresholds based on FRAX : A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos, 2016. 11(1): p. 25.
. For example, the FRAX® assessment takes no account of dose responses for several risk factors (such as glucocorticoid use, smoking, alcohol), and the number of prior fractures, or falls risk, should be considered.
To address some of the limitations of the original FRAX®, a new version of the fracture risk assessment tool, FRAXplus®, was released in 2023*.
While the FRAX calculator is available for free use, accessing FRAXplus adjustments requires the purchase of credits.
FRAXplus® allows adjustment of conventional FRAX® estimates of probabilities of hip fracture and major osteoporotic fracture (MOF) for the following factors:
- Recency of osteoporotic fracture according to the site
- Number of falls in the previous year
- High-dose exposure to oral glucocorticoids
- Duration of Type 2 diabetes mellitus
- Concurrent information on lumbar spine BMD
- Hip axis length (HAL)
- Information on Trabecular Bone Score (TBS)
For further details and to access FRAXplus®, visit https://www.fraxplus.org/frax-plus
- Kanis, J.A., et al., Interpretation and use of FRAX in clinical practice. Osteoporos Int, 2011. 22(9): p. 2395-411.
- Kanis, J.A., et al., A brief history of FRAX. Arch Osteoporos, 2018. 13(1): p. 118.
- Kanis, J.A., et al., A systematic review of intervention thresholds based on FRAX: A report prepared for the National Osteoporosis Guideline Group and the International Osteoporosis Foundation. Arch Osteoporos, 2016. 11(1): p. 25.
- Kanis, J.A., et al., Worldwide uptake of FRAX. Arch Osteoporos, 2014. 9: p. 166.
- International Osteoporosis Foundation: FRAX: Identifying People at High Risk of Fractures. 2009.
List of further references and reviews:
- On the IOF website
- On the FRAX website
REFERENCES
Shevroja E et al., Update on the clinical use of trabecular bone score (TBS) in the management of osteoporosis: results of an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), and the International Osteoporosis Foundation (IOF) under the auspices of WHO Collaborating Center for Epidemiology of Musculoskeletal Health and Aging. Osteoporos Int. 2023 34(9): p1501-1529.
Diez-Perez A, et al., Radiofrequency echographic multi spectrometry for the in vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organised by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO), Aging Clin. Exp. Res 2019; 31: p. 1375–1389.